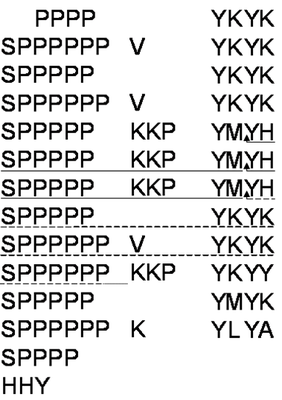Extensin
Probing the effects of extensin on primary cell wall properties.
The central role that primary cell wall plays in regulating extension growth and cell morphology requires a tight regulation of its rheological properties, which are ultimately determined by targeted changes in its composition and structure. The PCW lab. has been studying the effect of the structural protein, extensin, on cell wall properties.
Extensins have been reported as abundant in dicot primary cell walls (up to 10% w/w). These structural proteins have multiple sequence repeats (Fig. 1A) containing Ser(Hyp)4-6 structural motifs which promote a poly(II) pro-like configuration giving them an extended rod-like configuration reaching 50-80 nm in length (Cooper et al., 1987; Brownleader et al., 1996). Tyrosine and Lysine-containing motifs of extensins can be cross-linked by peroxidase and H2O2 to form a 3D protein network, thought to be essential for correct wall self-assembly and the definition of cell morphology and wall properties.
However, isolating the contribution of individual wall polymers to these fundamental changes in the wall nanostructure is difficult, especially over the time-scale required for developmental progression, where multiple changes can occur in the extracellular millieux.
We have chosen to utilise grapevine callus cell walls to model aspects of extensin function. The model system offers the means to manipulate the wall content of grapevine extensin (GvP1), which we have demonstrated to share many typical characteristics of dicot extensins, including proline hydroxylation and arabinoyslation (Fig. 1, right panel).
|
A |
B |
|
|
|
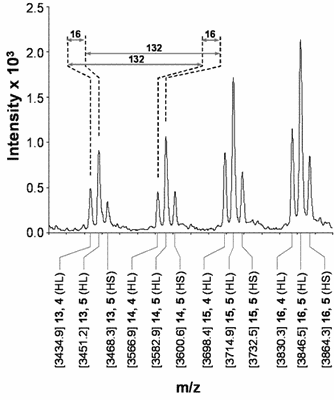
Fig. 1. Structural characterisation of GvP1 extensin. Left Panel: 5' truncated cDNA sequences of GvP1, showing its content of sequence repeats. The sequences underlined with a solid line represents P6, a peptide which can be isolated after CNBr fragmentation of GvP1. Right Panel: MALDI-TOF spectra of P6 shows heterogeneity in the hydroxylation (periodicity of 16) and arabinosylation (132) of GvP1.
Using this model system, we have recently obtained evidence that extensin network formation in these primary cell walls leads to a substantial reduction in wall hydration and width, with consequences to the shear stress required for microfibril reorientation during wall expansion (Fig. 2).
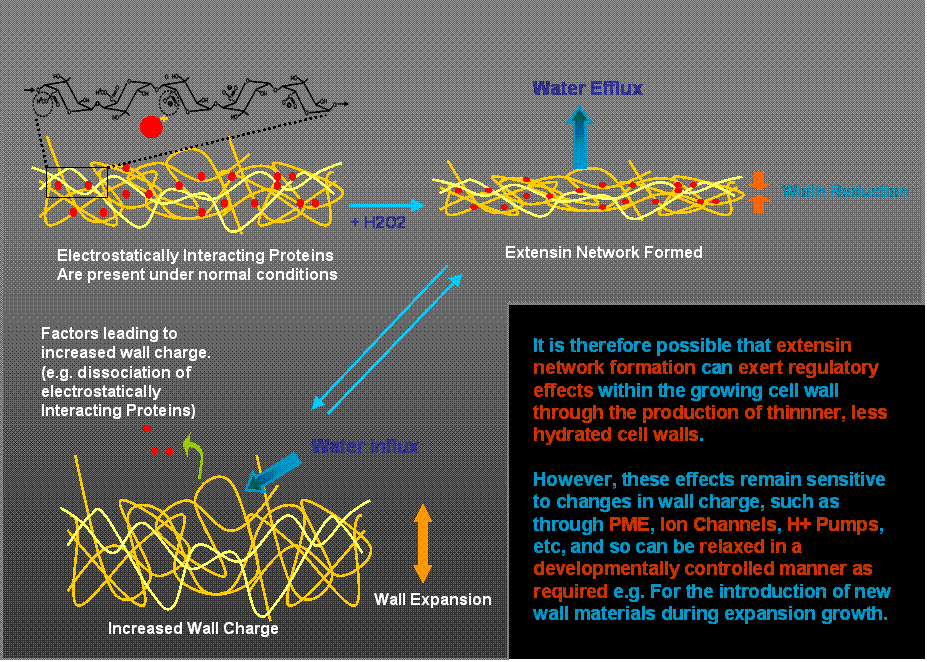
Fig. 2. Schematic representation of proposed extensin network effects on cell wall hydration and width. The proposal offers an explanation of how extensin can be involved in the early stages of wall biosynthesis (and self-assembly) to exert a regulatory influence on cell expansion, yet permit the developmentally regulated insertion of new materials required during expansion growth.