ABC transporters
Molecular modeling of ABC transporters: from ATP hydrolysis to substrate transport
The ATP-binding cassette (ABC) transporters are a super family of integral membrane proteins that are able to use the energy from ATP hydrolysis to actively drive substrates across lipid membranes against the concentration gradient. The substrates for these proteins are chemically very diverse, ranging from small ions, to sugars, amino acids, lipids, drugs, polysaccharides and even to polypeptides.
ABC transporters are one of the largest families known and its members are found virtually in all living organisms. The members of this family play important roles in several physiological processes such as the import of nutrients and the export of cytotoxic compounds from cells. Moreover, several members of the ABC family are associated with genetic diseases, such as cystic fibrosis, Tangier disease and to multidrug resistance in bacteria, fungi, yeasts, parasites and mammals.
Despite the transporters cellular functions, directionality and diversity of the substrate transported, all members of this family are composed by a minimal basic functional unit, consisting of a pair of highly hydrophobic trans-membrane domains (TMDs) and two cytoplasmic nucleotide binding domains (NBDs). The TMDs form a transmembrane pathway for the substrate whereas the NBDs are the catalytic motor domains where ATP hydrolysis occurs (Movie 1).
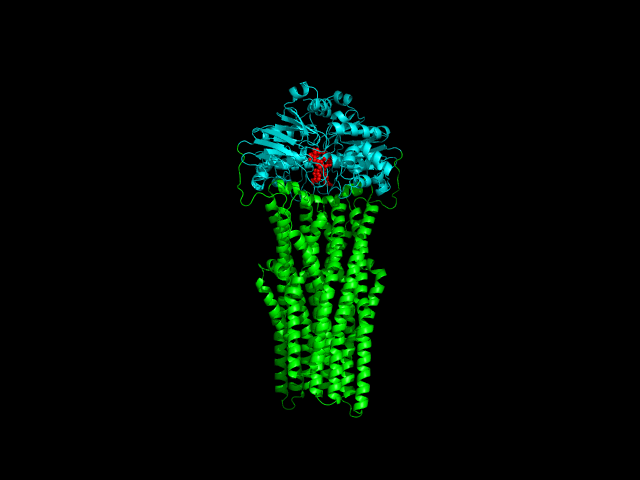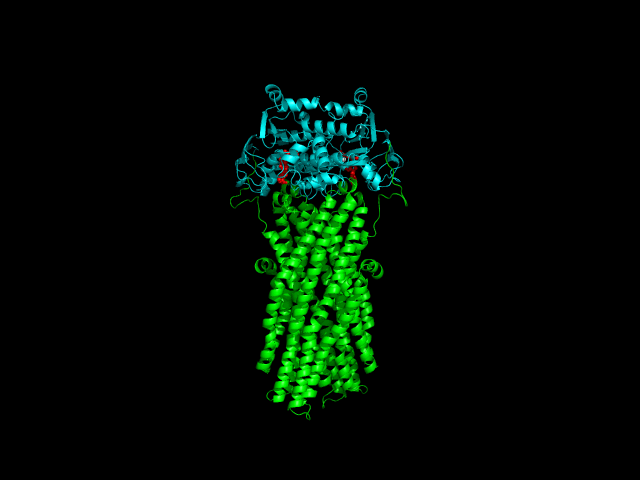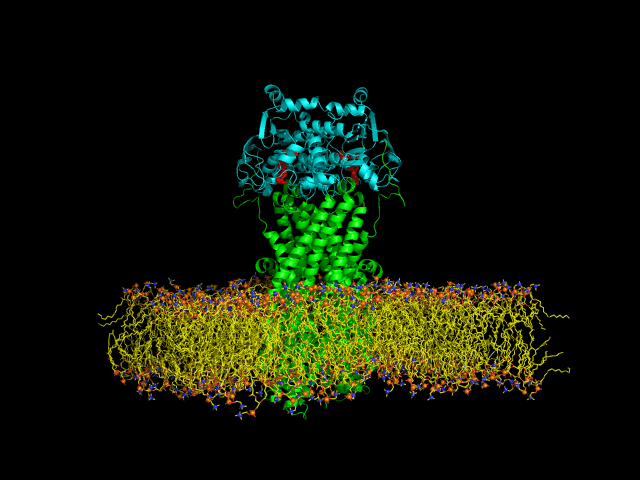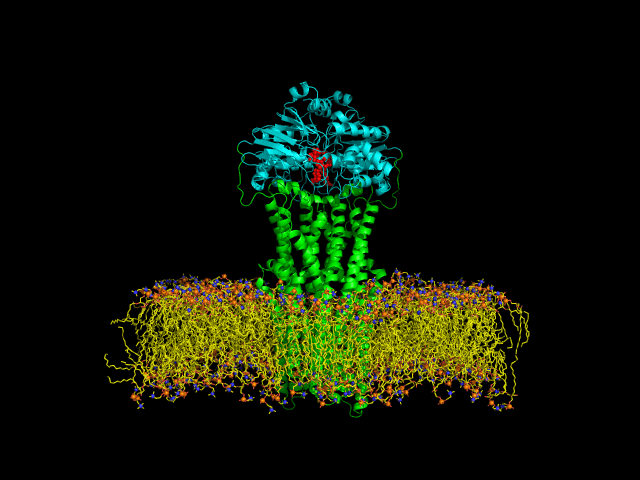
Until this moment, and despite the large amount of experimental and theoretical data available for several ABC members, many fundamental questions about the transport mechanism of these bio-nanomachines remain unanswered. In particular, it is not clear yet how the energy released from ATP hydrolysis is harnessed and transferred to the TMD, allowing the unidirectional transport of substrates through the membranes. In our laboratory, the main objective of this research project is to characterize the conformational rearrangements induced by the ATP-hydrolytic cycle and to elucidate how these atomic movements are transmitted from the catalytic to the transmembrane domains. For that purpose, we use theoretical techniques (mainly molecular dynamics simulations and continuum electrostatics) in order gain new insights into the ABC transporter working mechanism (movie 2 and movie 3).
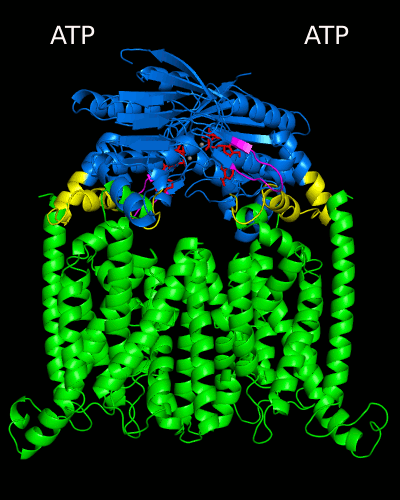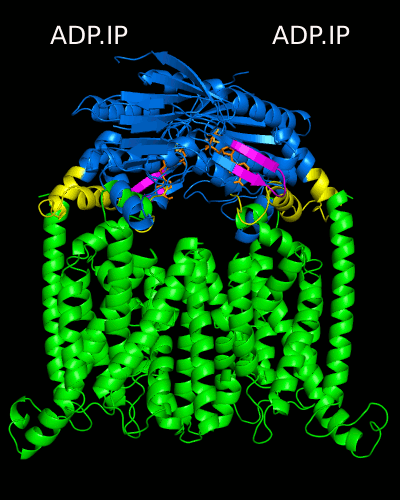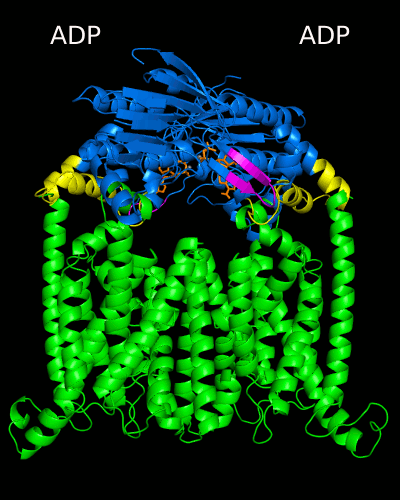
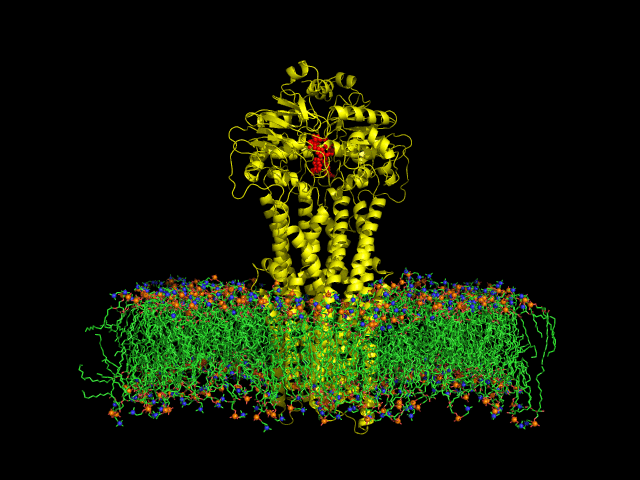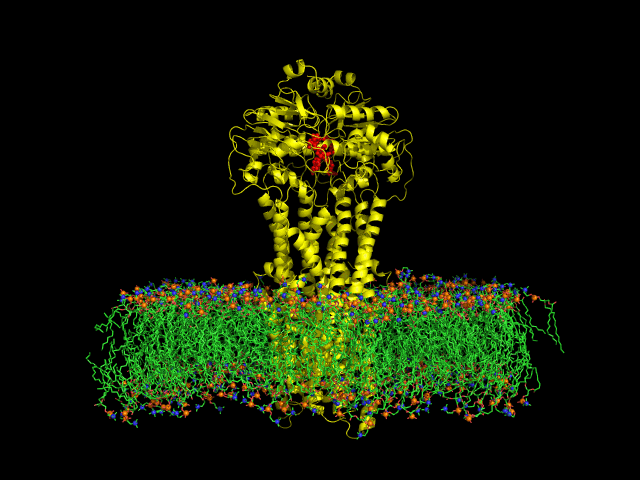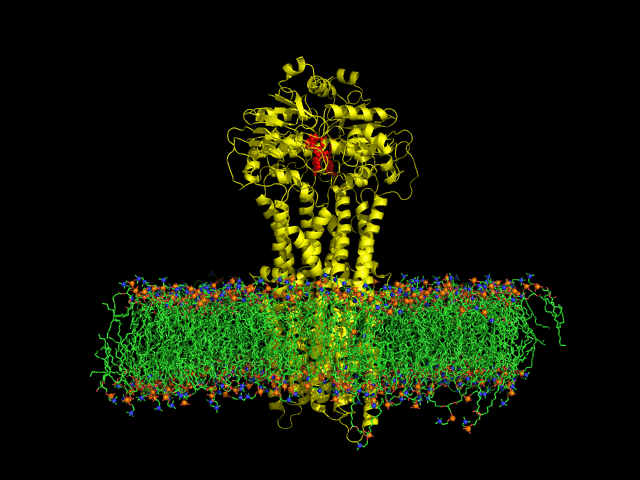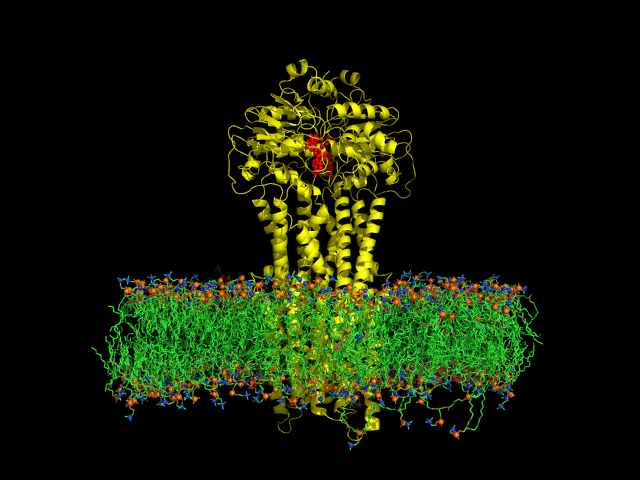
Recent relevant publications:
•Oliveira, ASF, Baptista, AM, Soares, CM (2011) “Inter-domain communication mechanisms in an ABC importer: a molecular dynamics study of the MalFGK2E complex”, PLoS Comp.Biol., 7(8), e1002128
• Damas, JM, Oliveira, ASF, Baptista, AM, Soares, CM (2011) “Structural consequences of ATP hydrolysis on the ABC transporter NBD dimer: molecular dynamics studies of HlyB”, Prot.Sci., 20, 1220-1230
• Oliveira, A.S., Baptista, A.M., Soares, C.M. (2011) " Conformational changes induced by ATP-hydrolysis in an ABC transporter: a molecular dynamics study of the Sav1866 exporter", Proteins, 79, 1977-1990
Oliveira, A.S., Baptista, A.M., Soares, C.M. (2010) " Insights into the molecular mechanism of an ABC transporter: conformational changes in the NBD dimer of MJ0796", J. Phys. Chem. B, 114, 5486-5496
• Roxo-Rosa, M., Xu, Z., Schmidt, A., Neto, M., Cai, Z.W., Soares, C.M., Sheppard, D.N., Amaral, M.D. (2006), "Revertant mutants G550E and 4RK rescue cystic fibrosis mutants in the first nucleotide-binding domain of CFTR by different mechanisms." PNAS, 103, 17891-17896



