A snapshot of sulfur bioenergetics
Oeiras, 14.06.09
Despite its toxicity, hydrogen sulfide can serve as electron donor in the respiratory chain of some microorganisms (and even animal mitochondria in certain conditions). One of the enzymatic systems known to make this possible is the sulfide:quinone oxidoreductase. ITQB researchers from the Laboratory of Membrane Protein Crystallography and the Laboratory of Metalloproteins and Bioenergetics have now determined the structure of this enzyme isolated from the membrane of the archaea Acidianus ambivalens, providing important insights into its catalytical mechanism. The results can be found online in the journal Biochemistry.
Adapted to live under very harsh conditions (pH 2 and 85 oC), in the presence of oxygen Acidianus ambivalens uses inorganic sulfur as the energy source, oxidizing it to sulfuric acid. Going from inorganic sulfur to sulfide requires the action of the SOR enzyme. Now, the structure for sulfide:quinone oxidoreductase (SQR) establishes the additional link with the respiratory chain and shows how A. ambivalens is able to extract as much energy as possible from inorganic sulfur.
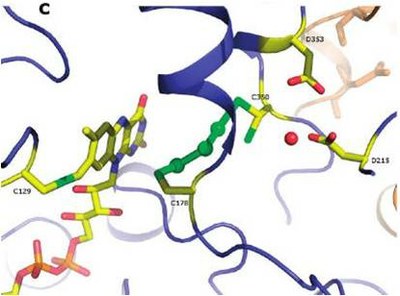
X-ray structure of SQR
|
After showing that A. ambivalens membranes consume oxygen in the presence of sulfide, researchers purified the enzyme, confirmed its sulfide:quinone oxidoreductase activity, obtained the protein crystals, submitted them to X-rays and analysed the diffraction pattern. This apparently straightforward process - essential for most structure determinations – can be long and bumpy, and is particularly challenging for membrane proteins. The X-ray structure obtained for SQR is a snapshot of an apparent intermediate state, with a chain of three sulfur atoms caught bridging two adjacent cysteine residues and reveals a second redox site with a covalently bound flavin. Resorting to protein modeling, since the C-terminal structure remained elusive, membrane anchoring is suggested to occur via an amphipatic helix located in that region. |
|
In the mechanism now proposed for sulfide:quinone oxidoreductase, the incoming hydrogen sulfide attacks the protein disulfide bridge and the sulfur is incorporated in an increasing polysulfide chain between the two redox cysteines; spatial constraints probably dictate the size of this chain and the polysulfide is eventually released. On the other hand, the electrons resulting from substrate oxidation are transferred to a quinone acceptor through the flavin cofactor, making the link with the respiratory chain.
Sulfur dependent bioenergetics
Besides proposing a mechanism for the catalytical sulfide oxidation, researchers further address the role of SQR in the sulfur dependent bioenergetics of A. ambivalens.
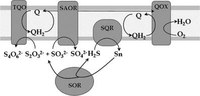 |
The initial step of sulfur metabolism is mediated by a soluble SOR that catalyzes the disproportionation of S0 to sulfite, sulfide, and possibly thiosulfate. Both sulfite and thiosulfate are further metabolized but part of the energy would be lost in sulfur reduction to sulfide. The presence of SQR, oxidizing sulfide (producing polysulfides/sulfanes that are again substrates for SOR) and reducing quinines, allows to extract maximum energy from S0, feeding electrons to the microorganism’s respiratory chain. |
Original Article:
Biochemistry, Article ASAP DOI: 10.1021/bi9003827
Structural and Functional Insights into Sulfide:Quinone Oxidoreductase
José A. Brito, Filipa L. Sousa, Meike Stelter, Tiago M. Bandeiras, Clemens Vonrhein, Miguel Teixeira, Manuela M. Pereira and Margarida Archer
J.A.B and F.L.S are equally contributing authors
Quote
The article has been highlighted in Faculty of 1000 Biology, an online research service that comprehensively and systematically highlights and reviews the most interesting papers published in the biological sciences.
Structural and Functional Insights into Sulfide:Quinone Oxidoreductase
Brito JA, Sousa FL, ..., Pereira MM, Archer M
Biochemistry 2009 May 28
“This article reports on the fascinating enzymology that underlies the ability of certain microorganisms and animal mitochondria to use hydrogen sulfide, a potentially toxic compound, as electron donor in the respiratory chain. Sulfide:quinone oxidoreductase uses a quinone as electron acceptor for the oxidative conversion of hydrogen sulfide to a polysulfide chain. The chemistry underlying this reaction is similar to that used by enzymes dealing with disulphide-containing molecules such as glutathione reductase or thioredoxin reductase. In particular, the enzyme makes use of a Cys-Cys bridge, which is located on a flexible loop. Hydrogen sulfide is proposed to attack this Cys-Cys bridge to generate, through various covalent intermediates, an increasingly longer polysulfur chain that is eventually released. The flexibility of the Cys-Cys loop is thought to enable the active site to host the elongating chain product. The electrons resulting from substrate oxidation are transferred to a quinone acceptor through a flavin
cofactor. The reaction occurs at the membrane surface so that the reaction products are released into the membrane bilayer and the reduced quinone can become available to the other respiratory enzymes. Very similar results have been obtained by Marcia et al. {1}.
References: {1} Marcia et al. Proc Natl Acad Sci USA 2009, Jun 1 Epub ahead of print [PMID:19487671].”