Best PhD Thesis at ITQB in 2009
Oeiras, 21.05.10
Ricardo Gouveia, former PhD student at the Laboratory of Glycobiology, was awarded the Best PhD Thesis Prize. The prize ceremony will take place on the ITQB Day, scheduled for July 9th 2010.
Supervised by Julia Costa, head of the Laboratory of Glycobiology, Ricardo Gouveia looked at the biological role of a particular glycoprotein, L1-CAM, a cell adhesion molecule critical for the development of the human central nervous system. To do this, he developed truncated versions of the protein that, among other applications in fundamental research and biotechnology, are useful as a screening tool for potential therapeutic antibodies against a particular form of human carcinomas. The research conducted in the last four years, and that resulted in a PhD Thesis, is published in five research articles. The PhD thesis is entitled "The neuronal L1 cell adhesion molecule – Production, characterization and biological role".
This award is attributed this year for the first time and aims to recognize the best PhD thesis presented at ITQB in 2009. The prize money of 2,500 Euros is funded by Fundação Jacqueline Dias de Sousa. The 2009 selection panel was chaired by Cândido Pinto Ricardo from ITQB.
The neuronal L1 cell adhesion molecule
Production, characterization and biological role
Ricardo Gouveia
This thesis is focused on the L1 cell adhesion molecule (L1-CAM), a glycoprotein critical for the development of the human central nervous system. L1 is expressed at the surface of neurons where it is concentrated in the growth cone. It is involved in numerous cellular functions, such as cell-cell adhesion, axon guidance, and neurite outgrowth. The functions of L1 are mediated through its interactions with a diverse set of molecules, including L1 itself (homophilic binding). Mutations in the L1 gene cause severe neurological disorders in neonates that include muscle spasticity, hydrocephalus and grave mental retardation.
L1 is a type I, membrane-bound glycoprotein that consists of six immunoglobulin-like domains, five fibronectin type III-like domains (ectodomain), a transmembrane region and a cytoplasmic tail. Several strategies to produce glycosylated mutants of recombinant human L1 have been explored in this work, using insect cells as hosts for stable expression. The proteins were produced in the milligram-per-liter range and increased levels were obtained by up-scaling the culture process [1]. Furthermore, the addition of dimethyl sulfoxide also led to increased levels of production [2]. The stable expression system using insect cells was found to be versatile, as it also produced an active form of a human glycosyltransferase [3].
The L1 mutants were shown to be correctly folded, as evaluated by a combination of far-UV circular dichroism and fluorescence spectroscopies. In addition, they were found to be glycosylated. As glycosylation is important for the function of L1, the N-glycans from L1 mutants were characterized by high-performance anion-exchange chromatography with pulsed-amperometric-detection, and by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. The major N-glycan was found to consist of the paucimannose structure Manα6(Manα3)Manβ4GlcNAcβ4(Fucα6)GlcNAc [2].
By using various L1 truncated mutants, it was possible to determine that the minimum part of the protein required for homophilic binding consisted of domains L1/Ig1-Ig4. Furthermore, the dissociation constants determined using surface plasmon resonance, were comparable for the homophilic interaction with the whole ectodomain or the mutant L1/Ig1-Ig4 [4]. Accordingly, L1/Ig1-Ig4 attached to cover slips was active in mediating cell adhesion and enhancing neurite outgrowth to a similar extent as the whole ectodomain. Finally, the results were compatible with a cooperative interaction between modules Ig1-Ig4 in a horseshoe conformation.
In conclusion, the work presented in this thesis contributed to the overall understanding of the function of L1, and particularly to the elucidation of the mechanism for L1 homophilic interaction. The use of L1 mutants can be envisioned in future applications, for fundamental research, in biotechnology, and in health. In particular, these mutants have already been applied in the screening of potential therapeutic antibodies against L1-expressing human carcinomas [5].
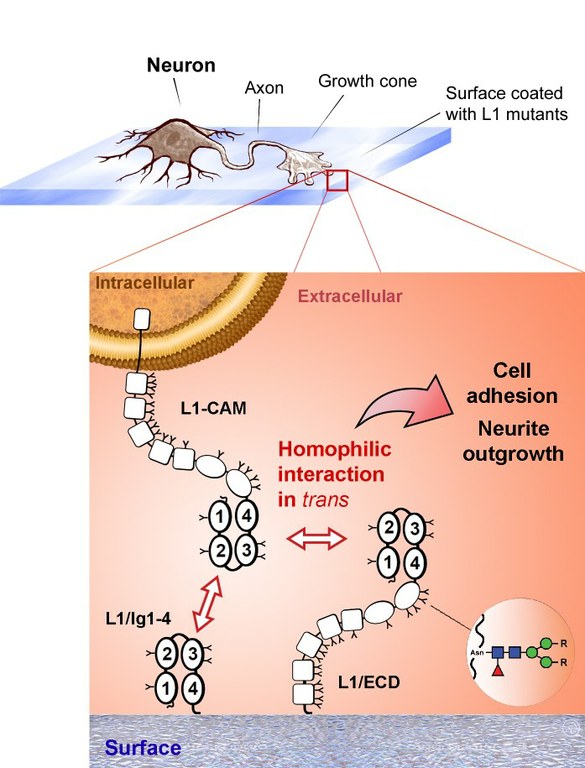 |
L1 domains Ig1-Ig4 are necessary and sufficient for L1 homophilic binding in trans. L1/ECD and L1/Ig1-4 produced in insect cells were shown to interact homophilicaly with native L1 from human neurons, thus promoting cell adhesion and enhancing neurite outgrowth. L1 mutants from insect cells were glycosylated, and contained mostly N-glycans of the paucimannose type (R=H). Blue square, N-acetylglucosamine; green circle , mannose; red triangle, fucose. |
References
[1] Gouveia, R.M., Morais, V.A., Peixoto, C., Sousa, M., Regalla, M., Alves, P.M. and Costa, J. (2007) Production and purification of functional truncated soluble forms of human recombinant L1 cell adhesion glycoprotein from Spodoptera frugiperda Sf9 cells. Protein Expr Purif 52: 182-193.
[2] Gouveia, R., Kandzia, S., Conradt, H.S. and Costa, J. (2010) Production and N-glycosylation of recombinant human cell adhesion molecule L1 from insect cells using the stable expression system. Effect of dimethyl sulfoxide. J Biotechnol 145: 130-138.
[3] Brito, C., Gouveia, R. and Costa, J. (2007) Stable expression of an active soluble recombinant form of human fucosyltransferase IX in Spodoptera frugiperda Sf9 cells. Biotechnol Lett 29: 1623-1630.
[4] Gouveia, R.M., Gomes, C.M., Sousa, M., Alves, P.M. and Costa, J. (2008) Kinetic analysis of L1 homophilic interaction: role of the first four immunoglobulin domains and implications on binding mechanism. J Biol Chem 283: 28038-28047.
[5] Wolterink, S., Moldenhauer, G., Fogel, M., Kiefel, H., Pfeifer, M., Luttgau, S., Gouveia, R., Costa, J., Endell, J., Moebius, U. and Altevogt, P. (2010) Therapeutic antibodies to human L1CAM: functional characterization and application in a mouse model for ovarian carcinoma. Cancer Res 70: 2504-2515.
Brief note on the awardee
Ricardo Gouveia graduated in Plant Biology from the Faculdade de Ciências da Universidade de Lisboa, in 2004. During the last year of graduation, he came to the ITQB to perform his undergraduate training at the Laboratory of Plant Genetic Engineering, under the supervision of Prof. Margarida Oliveira and Dr. Ana Sanchez. He then moved to the Laboratory of Glycobiology, first as graduate student, and later (2006-2009) as Ph.D. student, under the supervision of Dr. Júlia Costa, when he begun the study on the L1 protein. At present, he is post-doctoral fellow at the Laboratory of Glycobiology.









