Drug-target proteins mediate ribosome biogenesis, cell growth and proliferation
Oeiras, 08.06.2018
ITQB NOVA and iBET researchers, in collaboration with a French consortium composed by several labs, recently discovered novel insights about R2TP and R2TP-like complexes, multiprotein complexes that work as quaternary chaperones mediating ribosome biogenesis, cell growth and proliferation.
The work recently published in Nature Communications helps to understand how R2TP assembles and identified new protein partners, which form R2TP-like complexes.
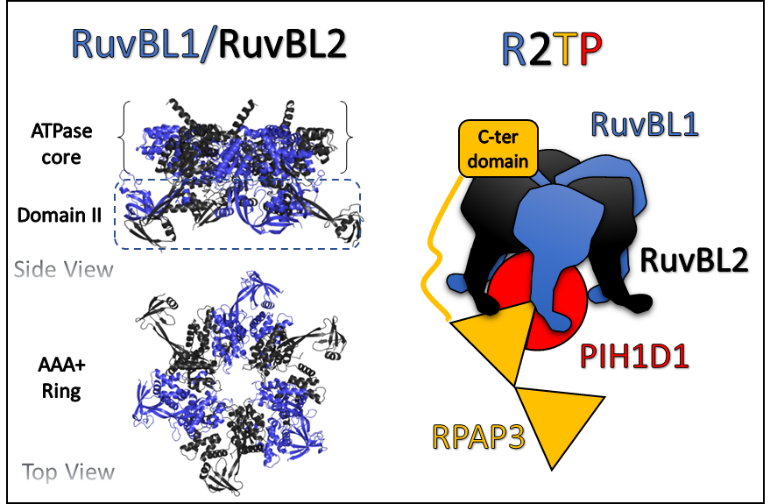
Heat shock protein 90 (Hsp90) is an abundant cytoplasmic chaperone, involved in cell signalling, proliferation and survival, and protein folding. In humans, it plays a role in neurodegenerative diseases and cancer. Human drug-targets ATPases RuvBL1/2 are constituents of R2TP complex, an Hsp90 quaternary co-chaperone involved in the assembly and maturation of multi-subunit complexes involved in gene expression and continuous cell proliferation.
RuvBL proteins are found in domains Archaea and Eukarya where they act in transcription regulation, DNA damage repair, cell cycle control, development, stress adaptation and disease. During crystallographic studies of human R2TP complex (composed of RuvBL1/2, RPAP3 and PIH1D1), using structural and interaction approaches, the teams from Structural Biology for Drug Discovery Lab (iBET, Merck Lab), the Industry and Medicine Applied Crystallography UNIT (ITQB NOVA) and the French consortium labs, explored a new role for the two ATPases (RuvBL1 e RuvBL2). RPAP3 protein was shown to directly bind RuvBL1/2 hexamer rings with strong affinity, providing insights into the complex architecture until now poorly known.
Given the roles of R2TP clients in cell growth and proliferation, this complex may mediate some of the tumorigenic effects elicited by Hsp90, therefore protein: protein interactions responsible for the stability of R2TP complex may be targeted in future therapeutic approaches.
The consortium includes the following institutions:
- IGMM, CNRS, Université de Montpellier, France
- Equipe labélisée Ligue Nationale Contre le Cancer
- IBSLor, Université de Lorraine, Nancy, France.
- iBET
- ITQB NOVA
- LSMBO, Université de Strasbourg, CNRS, France
- IMoPA, Vandœuvre-lès-Nancy, France
- CRBM, CNRS, Université de Montpellier, France
- IGF, CNRS, Université de Montpellier, France
- Hybrigenics Services, Paris France

Original Paper
Nature Communications (2018) DOI:10.1038/s41467-018-04431-1.
The RPAP3-Cterminal domain identifies R2TP-like quaternary chaperones
Chloé Maurizy1,2,+, Marc Quinternet3,+, Yoann Abel1,2, Céline Verheggen1,2, Paulo E. Santo4,5, Maxime Bourguet6, Ana C. F. Paiva4,5, Benoît Bragantini7, Marie-Eve Chagot7, Marie-Cécile Robert1,2, Claire Abeza1,2, Philippe Fabre7, Philippe Fort8, Franck Vandermoere9, Pedro M.F. Sousa4,5, Jean-Christophe Rain10, Bruno Charpentier7, Sarah Cianférani6, Tiago M. Bandeiras4,5, Bérengère Pradet-Balade8, Xavier Manival7, Edouard Bertrand1,2









