Strengthening bonds
Oeiras, 04.09.2015
A team of researchers from the Labs of Microbial Development, Membrane Protein Crystallography and Protein Modelling has characterized the structure and function of an ancient bacterial transglutaminase, an enzyme best known for its ability to cross-link proteins. The study is published in Biochemistry.
In living organisms, protein cross-linking by transglutaminases is central to a variety of processes in morphogenesis and development. More visible examples of protein cross-linking are clotting phenomena - the most famous transglutaminase is human blood coagulation factor XIIIa, which cross-links fibrinogen at wound sites. But transglutaminases confer chemical and mechanical resistance to macromolecular assemblies, organelles, and tissues in many organisms. In spore-forming bacteria, such as the bacterium Bacillus subtilis used in this study, transglutaminase cross-links the spore surface, contributing for the incredible resistance of these cell types.
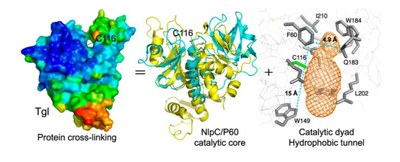
While the human and human-type transglutaminases are large, multidomain enzymes with complex activation mechanisms, the transglutaminase from Bacillus subtilis (Tgl) is a small, single domain enzyme, produced in active form and no factor is required for its activity – it is by far the smallest and simpler transglutaminase characterized to date. By analyzing the structure of Tgl, researchers have gained insight into the minimal structural requirements for protein cross-linking by transglutaminases. Furthermore, the structural and functional characterization of Tgl supports the idea that, irrespective of their evolutionary history, all transglutaminases have evolved from cysteine proteases.
Strikingly the catalytic cysteine is located in the center of an hydrophobic tunnel that transverses the molecule from side; the two protein substrates involved in the cross-linking reaction approach the active site trough the two entries of this tunnel. The ancient origin of Tgl, together with its small size, suggests that cysteine protease fold at the catalytic core, and the insulation of the active site from water during catalysis (so that isopeptide bond formation and not hydrolysis takes place), are essential requirements for protein cross-linking.
Transglutaminases catalyze cross-linking reactions between protein-bound glutamyl and lysil residues. Since the lysine substrate can be a small primary amine to which fluorphores, biotin, or other functional groups can be appended, Tgl is also an attractive tool for site-specific protein labeling in vitro and in vivo.
Original Article
Biochemistry (2015) Just Accepted Manuscript DOI: 10.1021/acs.biochem.5b00661
Structural and Functional Characterization of an Ancient Bacterial Transglutaminase Sheds Light on the Minimal Requirements for Protein Cross-Linking
Catarina G. Fernandes, Diana Plácido, Diana Lousa, José A. Brito, Anabela Isidro, Cláudio M. Soares, Jan Pohl, Maria A. Carrondo, Margarida Archer, and Adriano O. Henriques









