Shewanella oneidensis MR-1
Among the organisms capable of powering up MFCs, the dissimilatory metal reducing bacterium Shewanella oneidensis MR-1 (SOMR1) has been one of the most thoroughly studied. Extensive genetic and biochemical data have identified several multiheme cytochromes that are responsible for the reduction of insoluble metallic compounds. These include cymA, which encodes an inner membrane periplasmic tetraheme quinol dehydrogenase, and the OmcA-mtrCAB gene cluster which encodes two outer membrane decaheme cytochromes (OmcA and MtrC), a periplasmic decaheme cytochrome (MtrA) and a putative outer membrane beta-barrel protein (MtrB). A paralogue gene cluster mtrDEF encodes also a periplasmic decaheme cytochrome (MtrD), and a putative outer membrane beta-barrel protein (MtrE) and an outer membrane decaheme cytochrome (MtrF). Considering the different cellular locations of these cytochromes the Mtr pathway was proposed: electrons are transferred from the quinone pool in the cytoplasmic membrane via CymA to periplasmic cytochromes; these can be terminal reductases or redox proteins that mediate electron transfer across the periplasm to the outer membrane proteins MtrC, OmcA or MtrF that perform the actual extracellular electron transfer across the microbe-electrode interface, the last step of extracellular respiration. This group is interested in studying, in detailed, the electron transfer processes performed by these proteins.
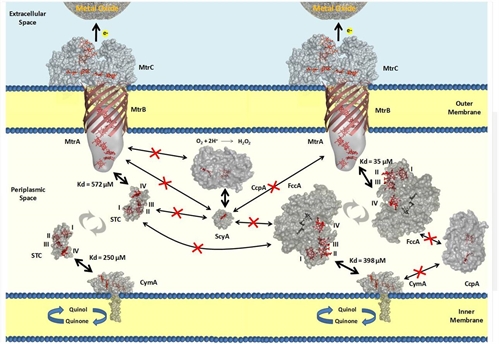
From Fonseca et al (2013) Biochem. J.






