Laccases and Metallooxidases
Laccases and Metallooxidases
Biomolecular Modelling and Structural Bioinformatics of Laccases and Metallooxidases, and their Molecular Mechanisms
Collaboration between the Laboratories of Lígia O. Martins, Isabel Bento and Cláudio M. Soares.
Financed project: Sixth Framework Programme (FP6-2004-NMP-NI-4) “BIORENEW: White biotechnology for added value products from renewable plant polymers: Design of tailor-made biocatalysts and new Industrial bioprocesses”
Laccases are a subgroup of the multicopper oxidase family of enzymes and can be found in fungi, plants, bacteria and even insects. They oxidise a wide variety of substrates, from aromatic organic molecules to metal ions, at the same time they reduce dioxygen to water. The oxidation as a process is very important from the industrial point of view. In the current days, many industrial processes, from lignin degradation in paper industry to the detoxification of textile industrial dyes, rely on oxidation through the use of harsh and toxic chemicals. Laccases may turn out to be viable substitutes for those chemicals, becoming more sustainable and environment-friendly solutions for those oxidation processes. By understanding the basic structural and functional molecular details of laccases, we may be able to optimize and engineer their stability and catalytic, as well as other, properties towards obtaining better biocatalysts.
Structurally, laccases are constituted by three cupredoxin-like motifs and possess two copper centres. Near the protein surface, there is a monocopper centre, called T1 copper centre, coordinated by two histidines and a cysteine and a methionine. Internalized in the protein, there is a tricopper centre, called T2-T3 copper centre, which possesses two T3 coppers coordinated by three histidines each and one T2 copper centre coordinated by two histidines. Catalytically, laccases are thought to receive electrons from a substract to the T1 copper centre through one-electron oxidations. Those electrons are then passed through a cystein-histidines triad to the T2-T3 copper centre which binds the dioxygen and reduces it to water.
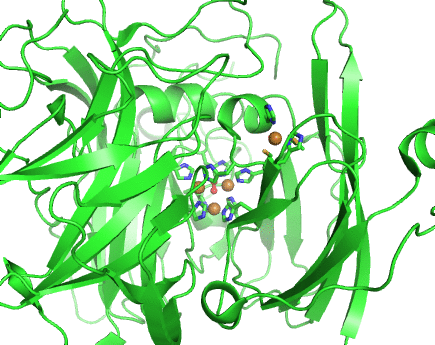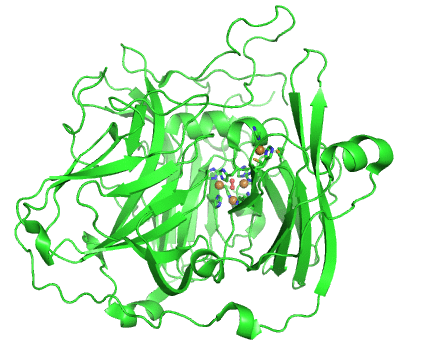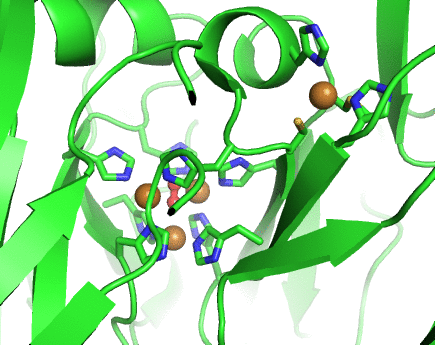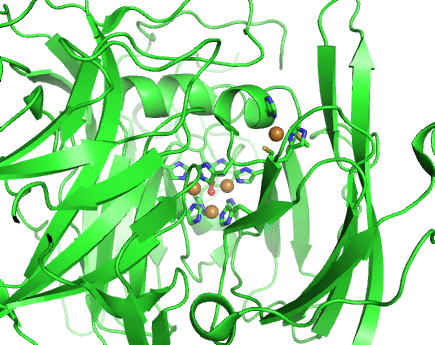
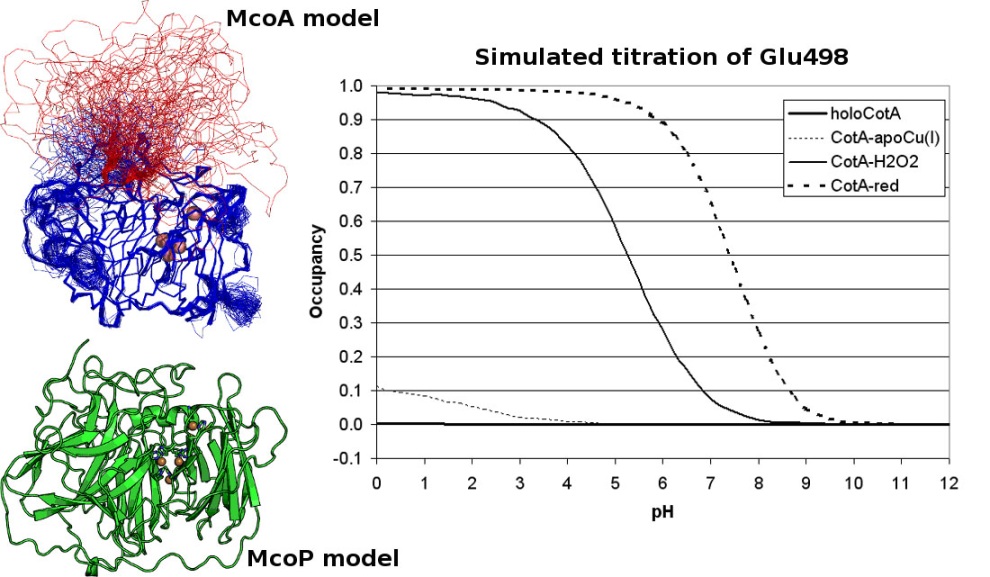
Working together with our collaborators from the enzymology and protein crystallography laboratories, we have studied the structural and catalytic properties of a laccase from B. subtilis, the CotA laccase, as well as other new laccases from thermophylic organisms. We have successfully derived homology models of McoA laccase from A. aeolicus (Fernandes 2007) and McoP from P. aerophilum (Fernandes 2010), and compared them structurally with CotA and CueO bacterial laccases in order to understand their structure-function relations. We have also performed protonation and redox calculations on CotA laccase structures in different steps of dioxygen reduction and revealed the importance of Glu498 for proton channeling to the T2-T3 copper centre (Bento 2010).
Furthermore, we are applying other molecular modelling approaches such as molecular docking, molecular dynamics and electron transfer prediction in order to study the oxidation of substrates by laccases. We have been focusing on the interaction between CotA laccase and ABTS, a small organic aromatic molecule which is efficiently oxidised by this enzyme. These studies may important for the establishment of protein residues crucial to the electron transfer, which may be tuned through directed mutagenesis.
Recent relevant Publications:
- Martins, LO, Soares, CM, Pereira, MM, Teixeira, M, Costa, T, Jones, GH, Henriques, AO (2002) "Molecular and biochemical characterization of a highly stable bacterial laccase which occurs as a structural component of the Bacillus subtilis endospore coat", J.Biol.Chem., 277, 18849-18859
- Fernandes, AT, Soares, CM, Pereira, MM, Huber, R, Grass, G, Martins, LO (2007) “A robust metallo-oxidase from the hyperthermophilic bacterium Aquifex aeolicus”, FEBS J., 274, 2683-2694.
- Durão, P, Chen, Z, Silva, CS, Soares, CM, Pereira, MM, Todorovic, S, Hildebrandt, P, Bento, I, Lindley, PF, Martins, LO (2008) “Proximal mutations at the type 1 copper site of CotA-laccase; spectroscopic, redox, kinetic and structural characterization of Ile494A and L386A mutants”., Biochemical J. 412, 339-346
- Fernandes, AT, Damas, JM, Todorovic, S, Huber, R, Pogni, R, Soares, CM, Martins, LO (2010) “The Multicopper Oxidase from the Archaeon Pyrobaculum aerophilum Shows Nitrous Oxide Reductase Activity”, FEBS J., 277, 3176-3189
- Bento, I, Silva, C, Chen, Z, Martins, LO, Lindley, PF, Soares, CM (2010) “Mechanisms underlying dioxygen reduction in laccases. Structural and modelling studies.”, BMC Struct. Biol. 2010, 10:28
- Silva, CS, Damas, JM, Chen, Z., Brissos, V, Martins, LO, Soares*, CM, Lindley, PF, Bento*, I (2012) “The role of Asp116 in the reductive cleavage of dioxygen to water in CotA laccase: assistance during the proton transfer mechanism”, Acta Crys D, D68,186-193.



