Do tree-like molecules fold?
Oeiras, 21.03.11
The Molecular Simulation Laboratory at ITQB has published the first detailed study of the conformational preferences of peptide dendrimers. The work, now on the Journal of the American Chemical Society, uses computational methods to investigate the until now elusive structure of these branched molecules.
Peptide dendrimers are synthetic molecules obtained by alternating standard amino acids with bifurcated diamino acids, thus producing a branched tree-like structure. These molecules have been used to mimic biological functions, such as enzyme activity, ligand binding, and drug delivery. Their biocompatibility and enhanced resistance to proteolysis makes peptide dendrimers promising for biomedical applications. But because all experimental attempts have been unable to reveal their three-dimensional structure, most reasoning about their structure-function features remains largely speculative, making impossible to follow a truly rational design. Now, thanks to the molecular simulation approaches used in this work things may change.
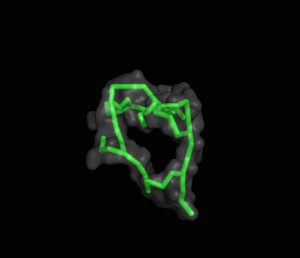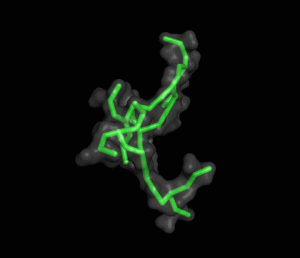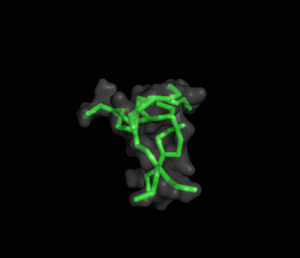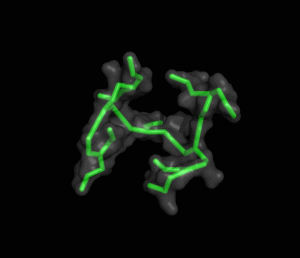 The ITQB researchers focused their study on a class of peptide dendrimers able to coordinate the cobalt atom of vitamin B12, which makes them potential B12 transporters to treat pernicious anemia. By conducting extensive molecular simulations of those dendrimers and some of its variants, the researchers characterized their conformational behaviour and inferred their affinity for vitamin B12. Unlike globular proteins, none of the studied dendrimers favours a preferential folded structure, displaying instead a very high conformational diversity. Still, two clearly distinct behaviours were observed in terms of compactness, apparently related to the flexibility of the branched amino acids. By relating these structural findings with the existing experimental data the researchers also proposed a new peptide dendrimer expected to have a higher affinity for vitamin B12.
The ITQB researchers focused their study on a class of peptide dendrimers able to coordinate the cobalt atom of vitamin B12, which makes them potential B12 transporters to treat pernicious anemia. By conducting extensive molecular simulations of those dendrimers and some of its variants, the researchers characterized their conformational behaviour and inferred their affinity for vitamin B12. Unlike globular proteins, none of the studied dendrimers favours a preferential folded structure, displaying instead a very high conformational diversity. Still, two clearly distinct behaviours were observed in terms of compactness, apparently related to the flexibility of the branched amino acids. By relating these structural findings with the existing experimental data the researchers also proposed a new peptide dendrimer expected to have a higher affinity for vitamin B12.
Overall, this study shows that peptide dendrimers have complex conformational behaviours that are not easily inferred from their chemical formula. Together with available experimental data, molecular simulation studies can play an important role in revealing the function-structure determinants of these molecules, ultimately leading to a more rational design.
Original Article
J. Am. Chem. Soc. (2011), article ASAP
Unfolding the Conformational Behavior of Peptide Dendrimers: Insights from Molecular Dynamics Simulations
Luís C. S. Filipe, Miguel Machuqueiro and António M. Baptista









