Areas of Research
-Biological Iron Uptake
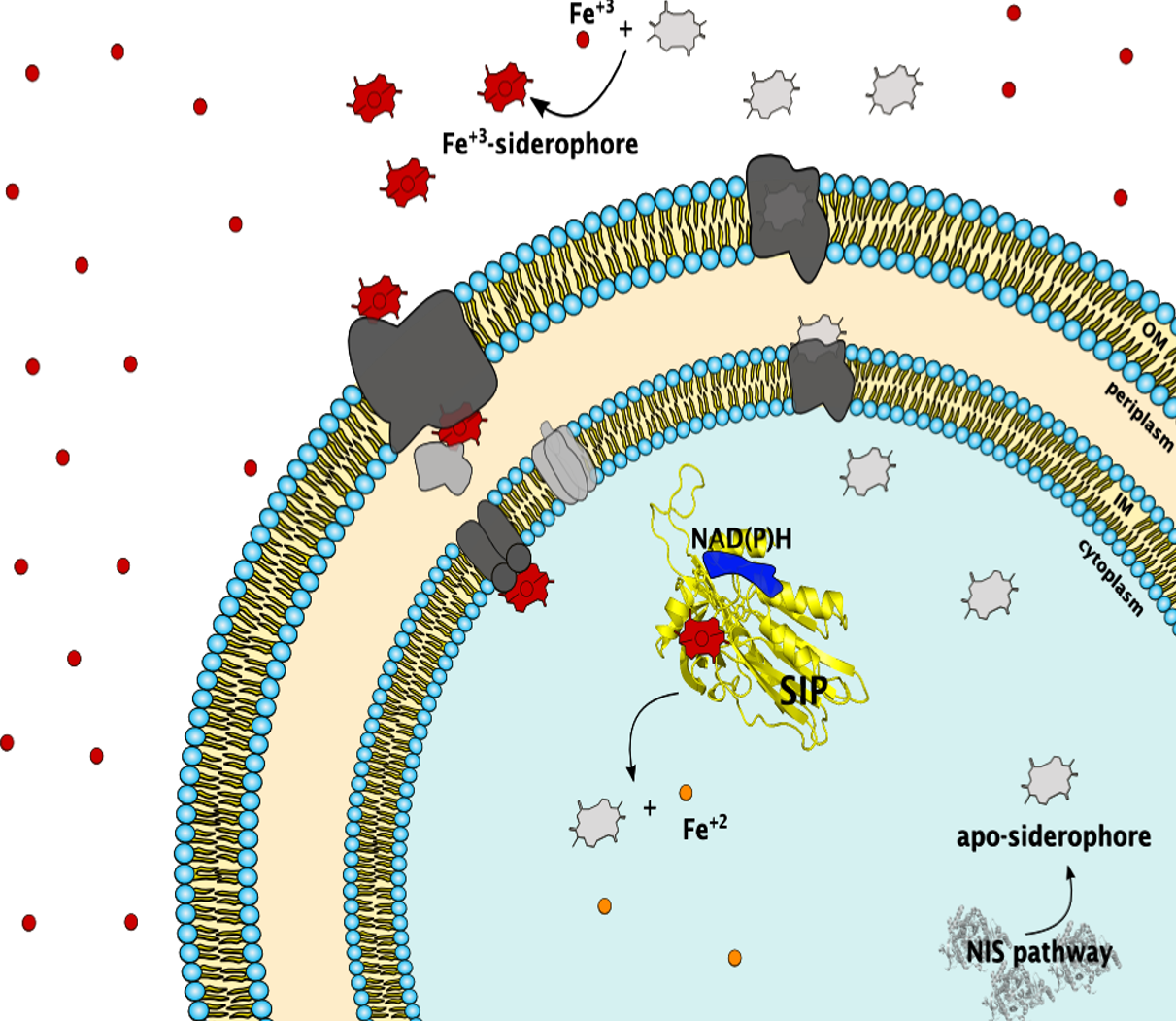
Iron is an essential element for Life although in oxgen-rich environments its bioavailability is limited. To circumvent this limitation organisms produce siderophores, small metal chelating compounds with high affinity for ferric iron. Siderophores are essential for the growth of marine microorganisms that form the bottom of the oceanic food chain, and for the proliferation of pathogens in iron depleted environments such as their eukaryotic hosts. Despite the broad relevance of siderophores for iron metabolism, within the siderophore pathway there is still limited knowledge on the molecular mechanisms of iron release from the ferric-siderophore complexes. In collaboration with colleagues we study these phenomena, using a multidisciplinary approach encompassing spectroscopy, structural biology, molecular genetics and infection biology. We have focused on the structural and functional characterization of ferric-siderophore reductases (FSRs) and the siderophore-interacting proteins (SIPs) from the Shewanella genus and E. coli. We also study the impact that these proteins have in opportunistic pathogen virulence using Zebra fish as model hosts. These studies aim to unravel the mechanism of action of FSRs and SIPs, and provide guidance for the development of strategies to stimulate oceanic productivity by enhancing iron uptake, and strategies to inhibit growth of pathogens by developing therapeutics that prevent iron uptake within their hosts.
Selected publications:
Trindade IB, Hernandez G, Lebègue E, Barrière F, Cordeiro T, Piccioli M, Louro RO, Conjuring up a ghost: Structural and functional characterization of FhuF, a ferric siderophore reductase from E. coli, J Biol Inorg Chem (2021) 26, 313-326, DOI: 10.1007/s00775-021-01854-y
Trindade IB, Silva JM, Fonseca BM, et al. Structure and reactivity of a siderophore-interacting protein from the marine bacterium Shewanella reveals unanticipated functional versatility. J Biol Chem. 2019;294(1):157-167. doi:10.1074/jbc.RA118.005041
-Metalloprotein maturation
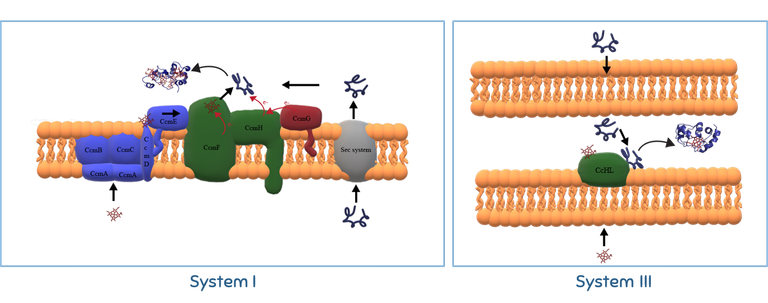
Metalloproteins such as cytochromes c require specific maturation systems to incorporate the co-factor after the synthesis of the polypeptide before they become functional. At IBN, we are interested in understanding cytochromes c maturation. The heme attachment in this important class of proteins does not occur spontaneously in nature, with a maturation system being essential for the process. Different organisms have different maturation systems and in the IBN laboratory we use a combination of methods in molecular biology, structural biochemistry and spectroscopy to investigate the molecular mechanisms of cytochrome c maturation used by the two most prevalent systems: Systems I and III. System I is present in Gram negative bacteria and, therefore, is of great relevance in the context of technologies falling under the broad definition of BioElectrochemical Systems. We have studies the molecular events leading to the maturation of cytochrome c’, a protein with substantial prevalence of alpha-helices and that is not expexted to rely on the heme attachement to establish those secondary structural elements. System III is present in eukaryotes, including humans, and is important in the context of human health. We have explored the substrate specificity of this maturation system to identify what elements in the sequence of the apo-protein are key to its recognition as an apo-cytochrome and key to promote covalent heme attachement.
-Biological electron transfer in vitro
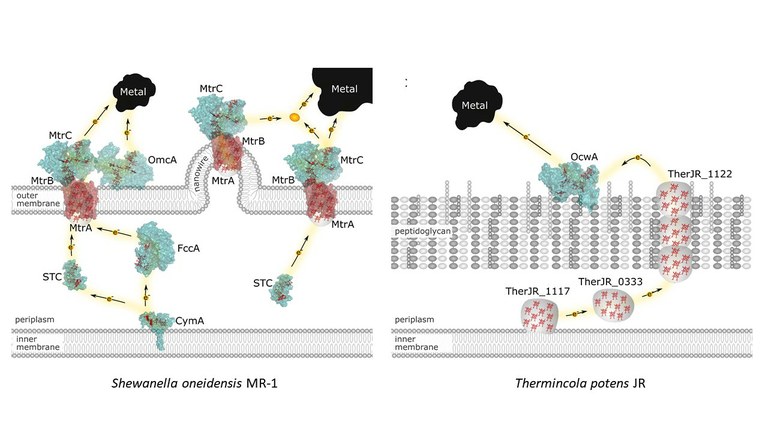
Electron transfer reactions in biological systems play essential roles in the metabolism. They are often coupled with other chemical reactions, and in particular for the bioenergetic metabolism, the coupling with proton transfer (the redox-Bohr) effect is essential for energy transduction. The study of these reactions requires a combination of biochemical, spectroscopic and electrochemical methods. We investigate the molecular mechanism employed by bacteria that generate or use electricity in their metabolism (electroactive bacteria). We have focused on the structural and functional characterization of multiheme c-type cytochromes found at the surface of these bacteria and that establish the electrical contact with the external circuit. We have also investigated the binding and recognition properties of cytochromes and other metalloproteins that participate in the metabolic pathways connecting the cell metabolism with the external circuit. This knowledge provides the compass to guide the development of biotechnological applications of these bacteria, with reduced ecological footprint.
Selected publications
Costa NL, Herman B, Fourmond V, Faustino MM, Teixeira M, Einsle O, Paquete C M, Louro RO, How thermophilic Gram-positive organisms perform extracellular electron transfer: characterization of the cell surface terminal reductase OcwA, mBio (2019) 10, e1210-19. DOI: 10.1128/mBio.01210-19
Paquete CM, Fonseca BM, Cruz D, Pereira T, Pacheco I, Soares CM, Louro RO, Exploring the molecular mechanisms of electron shuttling across the microbe/metal space, Frontiers Microbiol, 5:318, (2014) DOI: 10.3389/fmicb.2014.00318
Fonseca BM, Paquete CM, Neto SE, Pacheco I, Soares CM, Louro RO, Mind the gap: cytochrome interactions reveal electron pathways across the periplasm of Shewanella oneidensis MR-1, Biochem J, 449, 101-108 (2013) DOI:10.1042/BJ20121467
-Biological electron transfer in vivo
The study of biological electron transfer in vivo is fundamental to complement the in vitro studies, that often neglect important parameters, such as the cellular milieu that can influence the folding, stability and even the reactivity of proteins. Only with in vivo studies it is possible to fully understand the electron transfer processes that occur in biological systems, and elucidate the factors that control the electron transfer reactions. This knowledge in fundamental to manipulate redox proteins and improve electron transfer pathways in biological systems for specific purposes. At IBN we investigate the electron transfer processes performed by living electroactive bacteria, which can donate or receive electrons from electrodes. This is achieved by evaluating their capacity to reduce electrodes or decolorize the azo dye methyl orange that mimics functionally extracellular electron acceptors. We investigate the electroactive phenotype of deletion mutants to elucidate the role of specific redox proteins. We investigate the effect of specific mutations in redox proteins in the in vivo capacity of organisms for extracellular electron transfer. We also investigate the impact of transcription factors implicated in biolfim formation and stability in current production by electroactive bacteria.
Selected publications:
Silva AV, Edel M, Gescher J, Paquete CM, Exploring the effects of bolA in biofilm formation and current generation by Shewanella oneidensis MR-1, Front Microbiol 8, 815 (2020) DOI: 10.3389/fmicb.2020.00815
Fonseca BM, Silva L, Trindade IB, Moe E, Matias PM, Louro RO*, Paquete CM*, Optimizing electroactive organisms: the effect of orthologous proteins, Frontiers in Energy Research (2019) 7, DOI: 10.3389/fenrg.2019.00002
Delgado VP, Paquete CM, Sturm G, Gescher J, Improvement of the electron transfer rate in Shewanella oneidensis MR-1 using a tailored periplasmic protein composition, Bioelectrochemistry 129, 18-25 (2019) DOI: 10.1016/j.bioelechem.2019.04.022
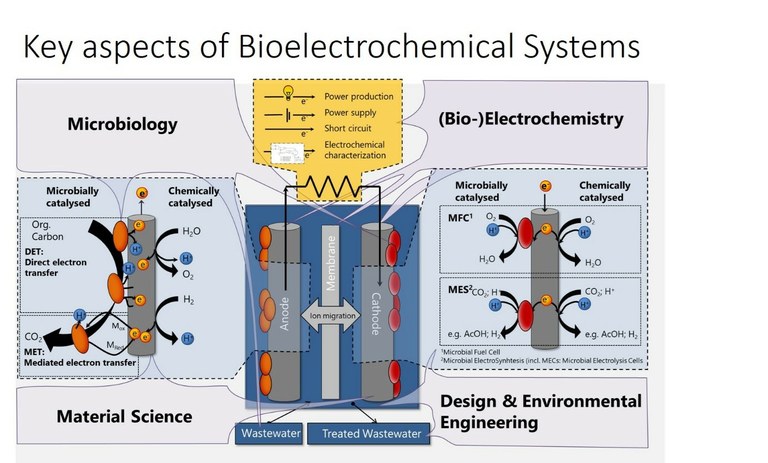
-Bioelectrochemical systems
Bioelectrochemical systems use the capacity of microorganisms or their enzymes to link their metabolism to electrical current. Based on this, a plethora of different applications raised in the bioelectrochemical field: sustainable energy production from wastewater in microbial fuel cells, bioproduction of different chemicals in microbial electrosynthesis, or soil remediation and analytical sensors. All of them can contribute to more sustainable industries and societies. Our contribution on their way to application is the investigation of different bacteria, materials, and reactors regarding their effect on the electron transport efficiency. Specifically we are investigating bioelectrosynthesis by acetogenic bacteria, and photobioelectrosysnthesis by purple bacteria, using poised cathodes as electron sources.
Selected publications:
Krieg T, Madjarov J, Rosa LMF, Enzmann F, Harnisch F, Holtmann D, Rabaey K, Reactors for Microbial Electrobiotechnology, Bioelectrosynthesis 167, 231-271 (2018) DOI: 10.1007/10_2017_40
-Paramagnetic NMR in structural biology
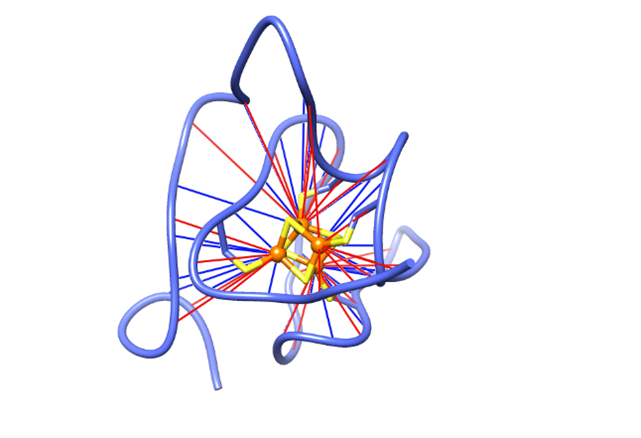
Metalloenzymes play key roles in biology performing electron transfer in bioenergetics pathways and redox linked catalysis of molecular transformations. Often the physiological or catalytically relevant states contain unpaired electrons. We develop methods for characterizing in detail the various paramagnetic states of multicentre redox proteins such as multiheme cytochromes, methods for the structural characterization of the active site of those proteins and enzymes and methods for the determination of the 3D structure of metalloproteins and enzyme mimetics using paramagnetic constrains.
Selected publications:
Maglio O, Chino M, Vicari C, Pavone V, Louro RO*, Lombardi A*, Histidine orientation in artificial peroxidase regioisomers as determined by paramagnetic NMR shifts, Chem Commun, 57, 990-993 (2021) DOI: 10.1039/D0CC06676A
Trindade IB, Invernici M, Cantini F, Louro RO*, Piccioli M*, PRE‐driven Protein NMR Structures: an Alternative Approach in Highly Paramagnetic Systems, FEBS J, 288, 3010-3023 (2021) DOI:10.1111/febs.15615
Neto SN, Fonseca BM, Maycock C, Louro RO, Analysis of the residual alignment of a paramagnetic multiheme cytochrome by NMR, Chem Commun 50, 4561 – 4563 (2014) DOI:10.1039/C3CC49135H
Fonseca BM, Saraiva IH, Paquete CM, Soares CM, Pacheco I, Salgueiro CA, Louro RO The tetraheme cytochrome from Shewanella oneidensis MR-1 shows thermodynamic bias for functional specificity of the hemes, J Biol Inorg Chem, 14, 375-385 (2009) DOI: 10.1007/s00775-008-0455-7