SOR
Sulfur Oxygenase Reductase
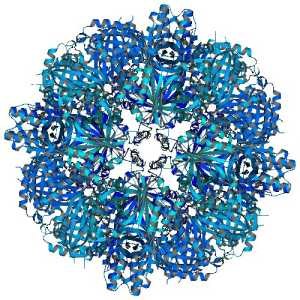 | |
| Fig. 1: The SOR holoenzyme. Cartoon representation viewed along the crystallographic fourfold axis. Note the protrusions at fourfold pseudo-symmetry axes. | Fig. 2: Substrate access and determinant residues in enzyme activity. (a,b) In silico modeling of a-S8 sulfur and linear polysulfide, respectively, represented by Van der Waals spheres into the 4-helix bundle “chimneys”, which lead to the interior of the sphere; gate-keeping residues in ball-and-stick representation |
SOR from the thermoacidophilic and chemolithotrophic archaeon A. ambivalens is the initial enzyme in its dissimilatory sulfur oxidation pathway. SOR is a self-compartment-alizing enzyme (308 aa) that disproportionates sulfur into H2S, SO32- and SSO32-, using a low-potential mono-nuclear non-heme iron centre as catalytic site, in a reaction where elemental sulfur works both as electron donor and acceptor.
References:
Urich T, Coelho R, Kletzin A, Frazao C. The sulfur oxygenase reductase from Acidianus ambivalens is an icosatetramer as shown by crystallization and Patterson analysis. Biochim Biophys Acta. 2005 Mar 14;1747(2):267-70.
Urich T, Gomes CM, Kletzin A, Frazão C. X-ray Structure of a self-compartmentalizing sulfur cycle metalloenzyme. Science. 2006 Feb 17;311(5763):996-1000.



