Conformational analysis is intended to give insight into the conformational characteristics of a flexible biomolecule and their relation to function. It can also be regarded as a first step of analysis that will point us to the structural properties of interest. However, conformational analysis can be a difficult task since proteins and peptides can adopt a myriad of different conformations.
A possible approach includes:
1) Finding a low-dimensional space that is able to maintain the distances among structurally (or kinetically) adjacent conformations.
2) Calculating the probability density function for this space. 3) Building the energy landscape and analysing its topology, which will give us "natural" conformational clusters corresponding to its basins (peaks in the Figure).
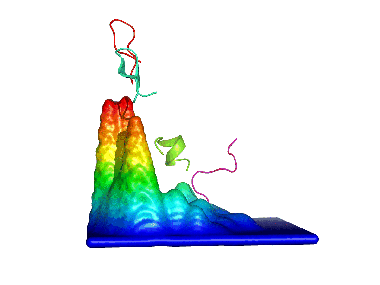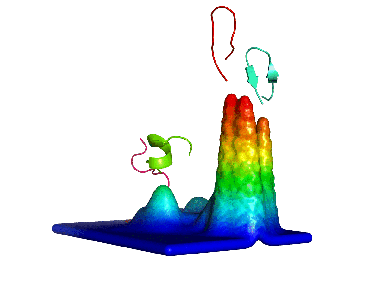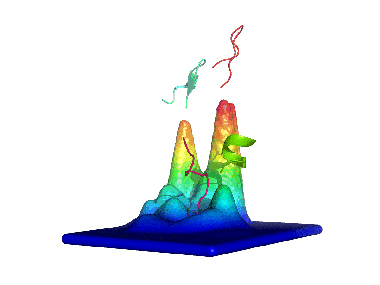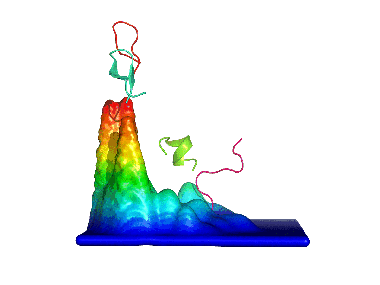
We first applied this method to the study of a decapeptide made of arginine/glutamate (RE) repeats which showed a very high conformational diversity (see here). Since then, we have been using it to analyze such diverse systems as the dipeptide kyotorphin, the prion protein (see here), and peptide dendrimers (see here and here).
We have performed an extensive conformational study of kyotorphin in water and membrane using this and other approaches.
Conformational study of kyotorphin Kyotorphin (KTP) was first isolated from bovine brain by Takagi and co-workers [1]. This endogenous dipeptide belongs to the neuropeptide family due to its opiate-like activity. KTP does not bind the opiate receptor but does have a met-encephalin releasing effect [2].
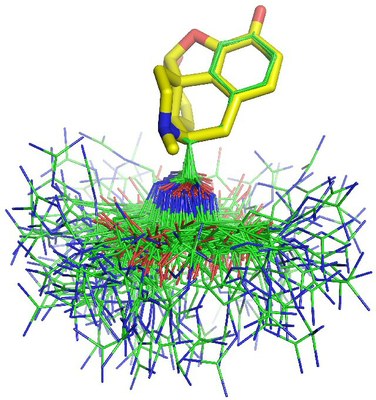
The study of KTP in water allowed us to elucidate the dipeptide preferred conformations at different pH values and how they correlate with known structural and functional information (see here). In the next Figure there is a fit of the 156 best conformations of KTP (lines; carbons in green) to the structure of morphine (sticks; carbons in yellow). All protons are omitted for clearness. The best fitted KTP conformations revealed that the Arg side chain does not invade the vicinities of the phenol group of the Tyr, allowing this to be presented to the receptor without any hindering effects, and therefore, there is no indication for the KTP receptor pocket to be very different from the ones of the opioid receptors.
We also studied KTP in the membrane with the N-terminus either charged or neutral (see here). We observed that one torsion (χ1 dihedral) was constrained in the membrane when KTP was in the charged form. This structural constraint is in agreement with known opioid ligand-receptor interactions, suggesting that the membrane stabilizes the conformers better suited for receptor binding. In the Figure below, we can see the superimposition of 180 conformations of charged KTP (taken at regular intervals) fitted by their main chain atoms. Unlike what happened in water, the membrane decreased the population of conformers with χ1 around 60°.

1. Takagi, H., et al. (1979) Eur. J. Pharmacol., 55, 109.
2. Takagi, H. et al. (1979) Nature, 282, 410.
Publications related with this theme 1. Machuqueiro, M., Baptista, A. M. (2007) The pH-dependent conformational states of kyotorphin: a constant-pH molecular dynamics study. Biophys. J. 92:1836-1845.[doi]
2. Campos, S. R. R., Baptista, A. M. (2009) Conformational analysis in a multidimensional energy landscape: study of an arginylglutamate repeat. J. Phys. Chem. B, 113:15989-16001.[doi]
3. Machuqueiro, M., Campos, S. R. R., Soares, C. M., Baptista, A. M. (2010) Membrane-induced conformational changes of kyotorphin revealed by molecular dynamics simulations. J. Phys. Chem. B, 114:11659-11667.[doi]
4. Filipe, L. C. S., Machuqueiro, M., Baptista, A. M. (2011) Unfolding the Conformational Behavior of Peptide Dendrimers: Insights from Molecular Dynamics Simulations. J. Am. Chem. Soc., 133:5042-5052.[doi]
|





