Hydrogenases
Hydrogenases
Hydrogenases
Collaboration between the Laboratories of Inês Cardoso Pereira, Pedro Matias and Cláudio M. Soares.
Financed project: FCT project PTDC/BIA-PRO/70429/2006. “Towards biohydrogen production – study of a high-activity, oxygen-resistant bacterial hydrogenase”.
Hydrogenases are enzymes that catalyze the oxidation of H2 to protons as well as the inverse reaction. The metabolic functions of the enzymes belonging to this group are very diverse. Some organisms contain hydrogenases that oxidize H2 and transfer the electrons to the respiratory chain, which allow these organisms to use H2 as an energy source. That is the case of the sulfate reducing bacteria Desulfovibrio vulgaris (Fig.1). Other organisms have hydrogenases that produce H2 for disposal of excess of reducing equivalents.
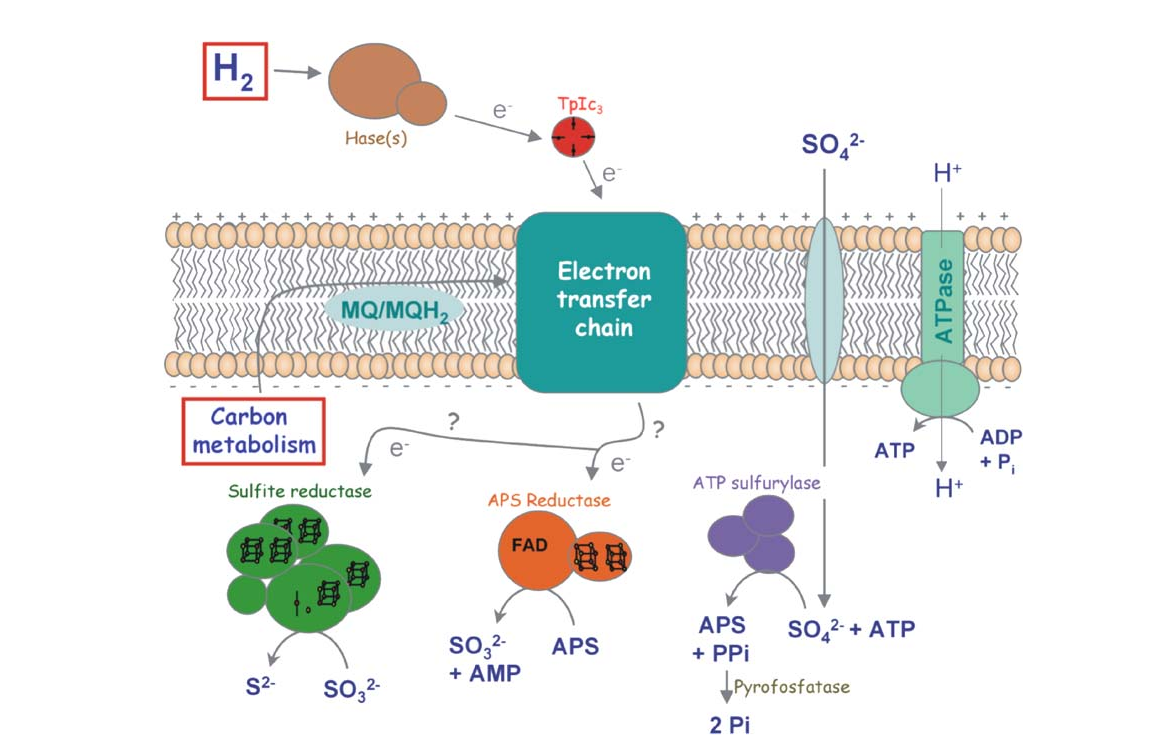
H2 is an attractive alternative to fossil fuels, but the currently available processes for producing it or for using its energy are not sustainable at a global scale. Microorganisms, and the hydrogenases they possess, could provide us solutions for these problems, either by using them directly or as an inspiration for new synthetic systems. One of the obstacles for the application of hydrogenases is the O2 inhibition.
The two major classes of hydrogenases are the [NiFe] and the [FeFe] hydrogenases. [NiFe] hydrogenases are more active in the oxidation of H2, and the [FeFe] hydrogenases are more active in the production of H2. The [NiFe] hydrogenases have received more attention because their inhibition by O2 is reversible, while in the [FeFe] hydrogenases it is irreversible.
The hydrogenases of the [NiFe] class are globular proteins with a deeply buried active site at its center (Movie 1). The active site has an iron and nickel ions connected to the protein by four cysteine residues. Because the active site is deeply buried the substrates/products have to cross the protein matrix to go from the active site to the solvent and vive-versa. Three iron-sulfur clusters allow the electron transfer between the active site and the redox partners. H2 diffuse through hydrophobic channels and protons are transfered between protonable residues and water from the active site to the solvent. These pathways are important in the regulation of the enzyme function as well as the control of inhibitors like O2.
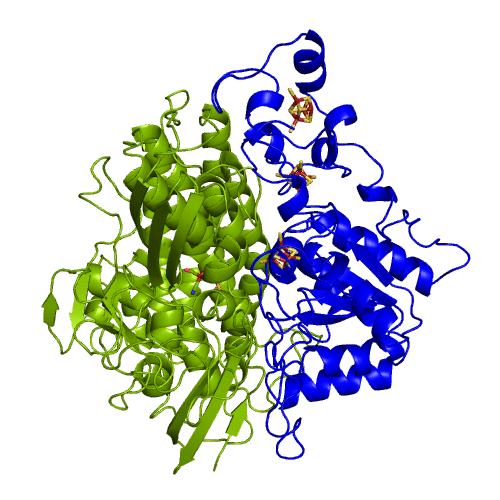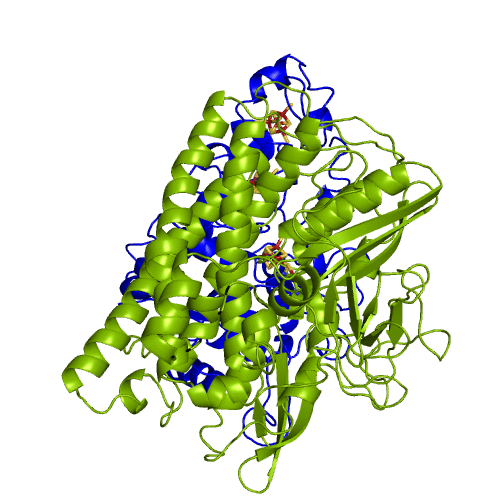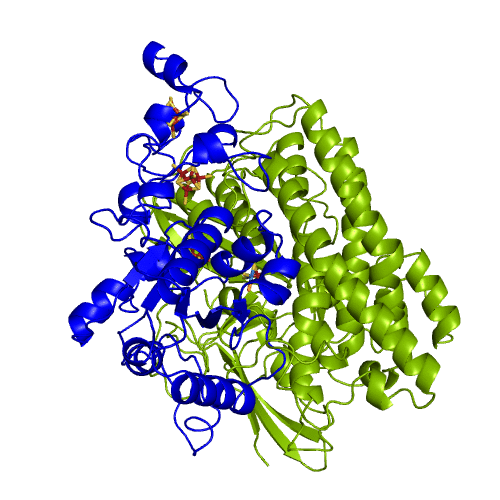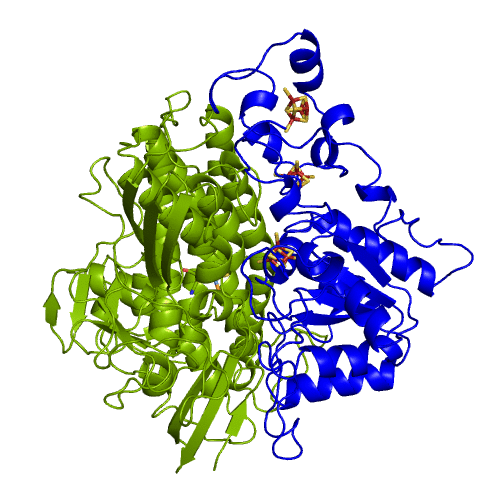
[NiFeSe] hydrogenases are a subgroup of [NiFe] with especially interesting properties for applications. In these hydrogenases, one of the cysteines that coordinate the nickel is replaced by a selenocystein. When compared to the standard [NiFe] hydrogenases, they have higher activities in the H2 production and are more tolerant to O2 inhibition. This is not fully understood, because according to the existent studies, the replacement of the cysteine is not sufficient to explain the functional differences between the two types of enzymes.
In our studies, we use different techniques: molecular dynamics simulation to characterize the H2 pathways (Movie 2) and continuum electrostatics is used to study the proton pathways (Fig. 2).
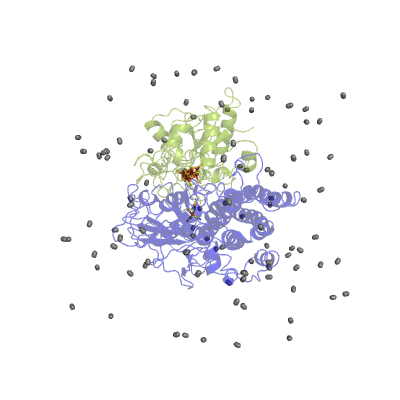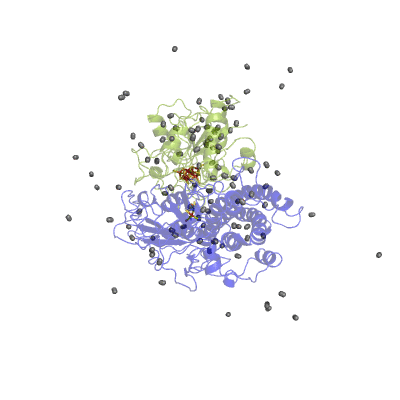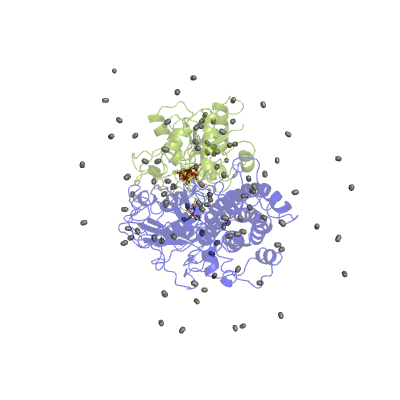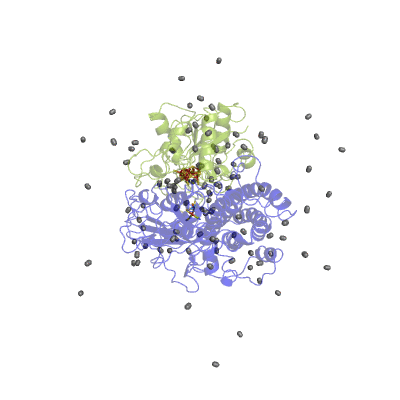
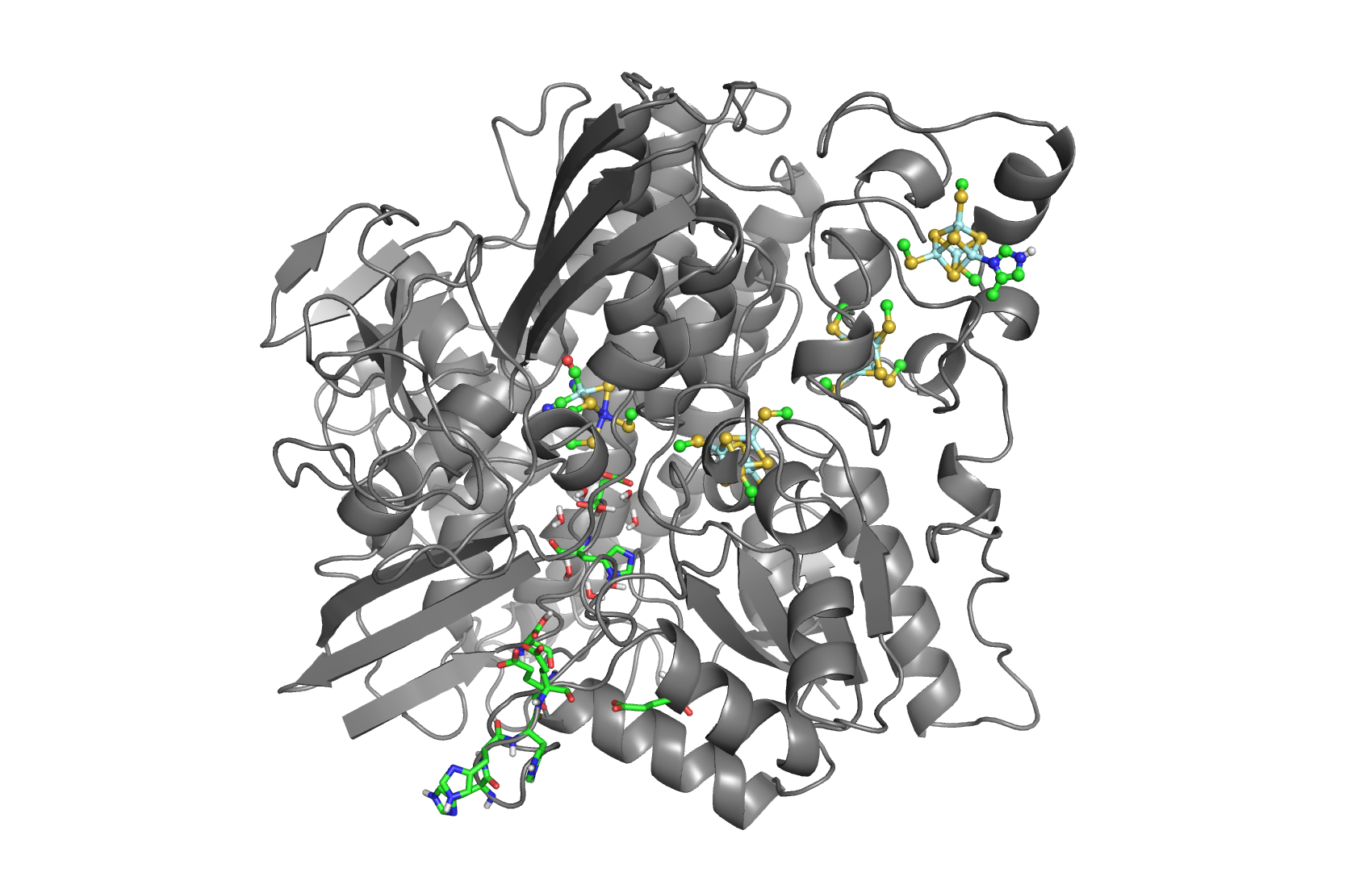
Figure 2 Proton pathways in a [NiFe] hydrogenase.
Recent relevant publications:
• Matias, P.M., Pereira, I.A., Soares, C.M., Carrondo, M.A. (2005) "Sulphate respiration from hydrogen in Desulfovibrio bacteria: a structural biology overview.", Prog. Biophys. Mol. Biol., 89, 292-329
• Valente, F.M.A., Oliveira, A.S.F., Gnadt, N., Pacheco, I., Coelho, A.V., Xavier, A.V., Teixeira, M., Soares, C.M., Pereira, I.A.C. (2005) "Hydrogenases in Desulfovibrio vulgaris Hildenborough: structural and physiologic characterisation of the membrane-bound [NiFeSe] hydrogenase", JBIC, 10, 667-682
• Teixeira, V.H., Baptista, A.M., Soares, C.M. (2006) "Pathways of H2 towards the active site of [NiFE]-hydrogenase", Biophys.J., 91, 2035-45
• Teixeira, V.H., Soares, C.M., Baptista, A.M. (2007) “Proton Pathways in a [NiFe]-Hydrogenase: A Theoretical Study", Proteins, 70, 1010-1022
• Baltazar, C, Marques, MC, Soares, CM, DeLacey, AM, Pereira, IAC, Matias, PM (2011) “Nickel-Iron-Selenium Hydrogenases - An Overview”, Eur.J.Inor.Chem., 2011, 948-962.
• Baltazar, CSA, Teixeira, VH, Soares, CM (2012) “Structural features of [NiFeSe] and [NiFe] hydrogenases determining their different properties: a computational approach”, JBIC, 17, 543-555



