Protein models
Polyphosphate kinase 2 from Deinococcus radiodurans (PPK2)
Polyphosphate kinase 2 catalyses preferentially the polyphosphate (polyP)-driven synthesis of ATP from ADP, and ADP from AMP. This enzyme has high value for biocatalysis, as it is very efficient in the regeneration of the ATP pool.
D. radiodurans contains very large electron dense granules rich in phosphate and selected cations, such as Manganese and Calcium. It is hypothesized that these are actually polyphosphate granules, and may be highly involved in this organism's exceptional resistance to radiation insult.
In Romão's Team (Structural Genomics Lab), we study several of the mechanisms used by D. radiodurans to protect the membrane, proteins and genomic material from reactive oxygen species that lead to oxidative stress.
Polyphosphate kinases are the main enzymes involved in polyP metabolism, and as such are an important player in the regulation of responses to oxidative stress and energetic needs of the cell.
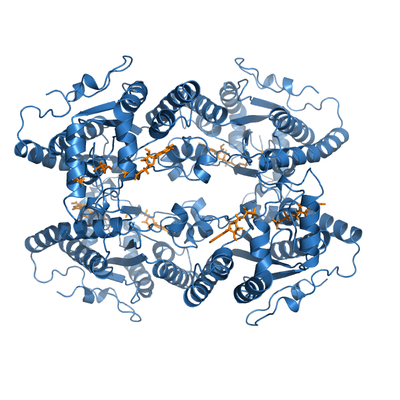
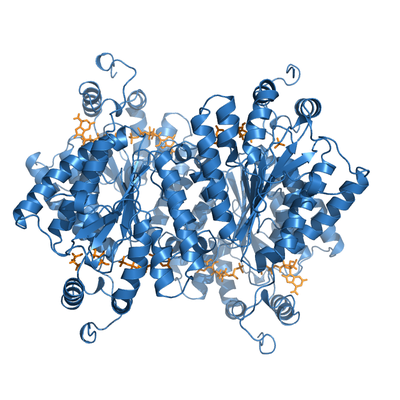
ADP-bound PPK2. This protein froms a crystallographyc tetramer, with two nucleotides bound per monomer (yellow).
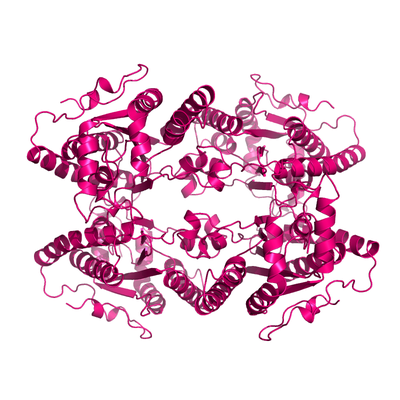
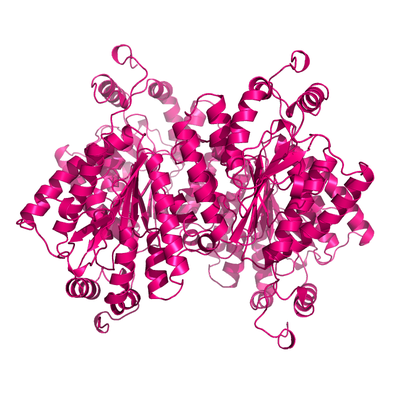
Apo form of PPK2.
Human RuvB-Like 2 (RuvBL2, Reptin)
RuvB-Like 2 is a human protein involved in multiple pathways, notably in early development. This protein is widely distributed in the organism, but particularly expressed in the testes. When disregulated, it is involved in cancer onset, metastisation and resistance of cancer cells to radiation therapy, possibly through the same mechanisms that regulate cell survival in hypoxic conditions.
My PhD work focused on RuvBL proteins, particularly RuvBL2. I used X-ray crystallography to determine the 3D model of RuvBL2, in the group of Pedro Matias (Industry and Medicine Applied Crystallography). One of the main outcomes of this work was the proposition of an action mechanism able to connect ATP binding to mechanical movement of Domain 2 (in green). This proposal has been since supported by subsequent studies from other groups, particularly in larger complexes such as R2TP.
RuvBL2 has ATPase activity, and works mainly as a chaperone in the assembly of large complexes, such as R2TP. In some types of cancer it was found to be overexpressed, which constitutes a poor prognosis.
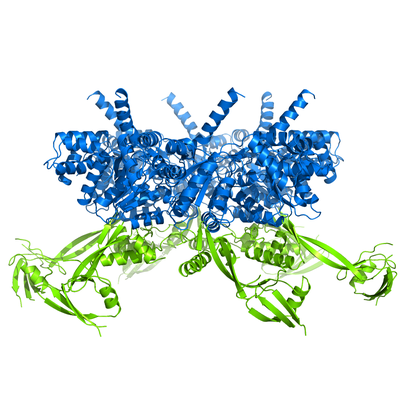
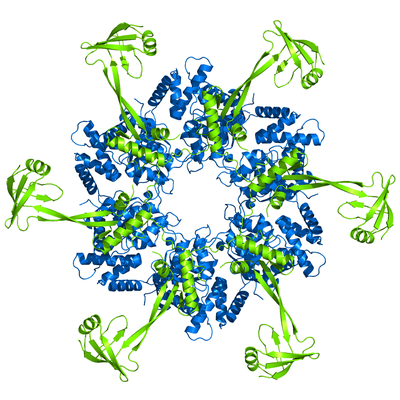
RuvBL2 hexamer side view (left) and top view (right). In blue is the ATPase core, and in green the regulatory Domain 2.



