I - O que nós estudamos.
1-Cadeias respiratórias e proteínas de transferência electrónica
One of our research lines concerns the study of electron transfer chains. Electron transfer chains couple electron transfer to proton translocation through the plasma membrane or the inner mitochondrial membrane, in prokaryotes or eukaryotes, respectively. A schematic representation of electron transfer chains in general is depicted in Figure 1.
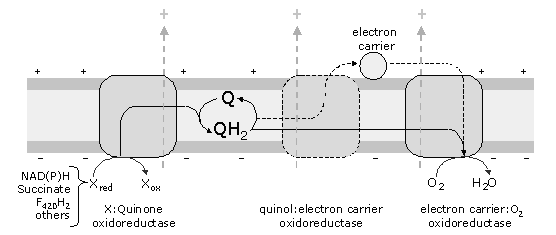
Figure 1 - General schematic representation of aerobic electron transfer chains. These chains receive electrons from different donors, such as NAD(P)H, succinate, F420H2 , electron transfer protein, glycerol 3-phosphate and others. The oxidation of these substrates is performed by oxidoreductases, which reduce quinones. The quinols, thus formed, may be directly oxidised by the oxygen reductases or trough intermediate complexes, which reduce metalloproteins (electron carriers, such as cytochrome c , HiPIP).
These chains may contain several enzymes that accept electrons from the so called electron donors or reducing equivalents (like NAD(P)H, succinate, F420H2 , glycerol-3-phosphate), and reduce quinones. At the end of the aerobic chains an oxygen reductase must be present. Other intermediate complexes, such as a quinol:electron carrier oxidoreductase may also be present. The best characterised electron transfer chains, those from mitochondria and related bacteria, are mainly composed of four complexes (named I to IV):
| Complex I - NADH:quinone oxidoreductase |
| Complex II - Succinate:quinone oxidoreductase |
| Complex III - Quinol:cytochrome c oxidoreductase |
| Complex IV - Cytochrome c : oxygen oxidoreductase |
2-Destoxificação de Superóxido e NO
We also aim at the study of metalloproteins involved in the prokaryotic response against oxidative and nitrosative stress.
Organisms growing in anaerobic environments (see Oxidative Stress and Anaerobes) are often challenged with the transient exposure to molecular oxygen and to other reactive oxygen species (ROS). The reductive cell environment quickly converts oxygen to its partially reduced, highly reactive and cytotoxic species, such as the superoxide anion and hydrogen peroxide. These organisms have thus developed, throughout evolution, a multitude of proteins for the detoxification of ROS: catalases and peroxidases for hydrogen peroxide and superoxide dismutases and superoxide reductases for superoxide. In our group, neelaredoxin and desulfoferrodoxin from several sources are studied as part of the defense mechanism against superoxide, acting as superoxide reductases.
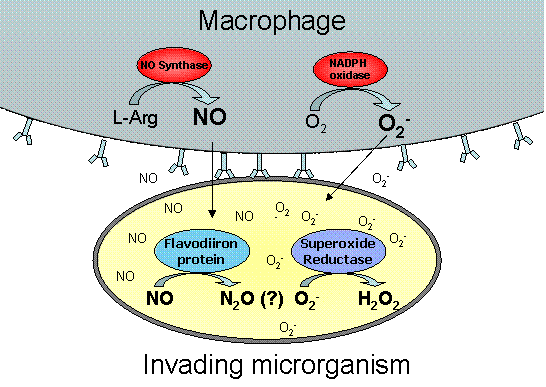
Figure 2- Some defense systems used by pathogens against superoxide and NO. Superoxide and NO are toxic radicals produced by macrophages to kill microrganims upon infections. However, some pathogens have developed strategies to defend themselves against this attack, by using enzymes that eliminate these toxic species, and thus being able to survive.
Another aspect of oxidative and nitrosative stress concerns the mammalian immunological defence mechanisms against pathogen invasion and infection. Activated macrophages are known to produce nitric oxide, a highly reactive and cytotoxic radical molecule which inhibits and destroys several cellular functions. Pathogens have thus evolved counteracting mechanisms to subvert the host's immunitary system, namely through the scavenging of nitric oxide. Flavodiiron proteins have recently been demonstrated to act as nitric oxide reductases, working as NO scavengers, alongside with the NO dioxygenase flavohemoglobin . In our group, we study the relevance of flavodiiron proteins from several organisms in the subversion of the immunitary system.