Projects
> Bacterial disease
> Calcium-binding proteins
> G-quadruplexes
> New methods
People
Collaboration
Funding
Publications
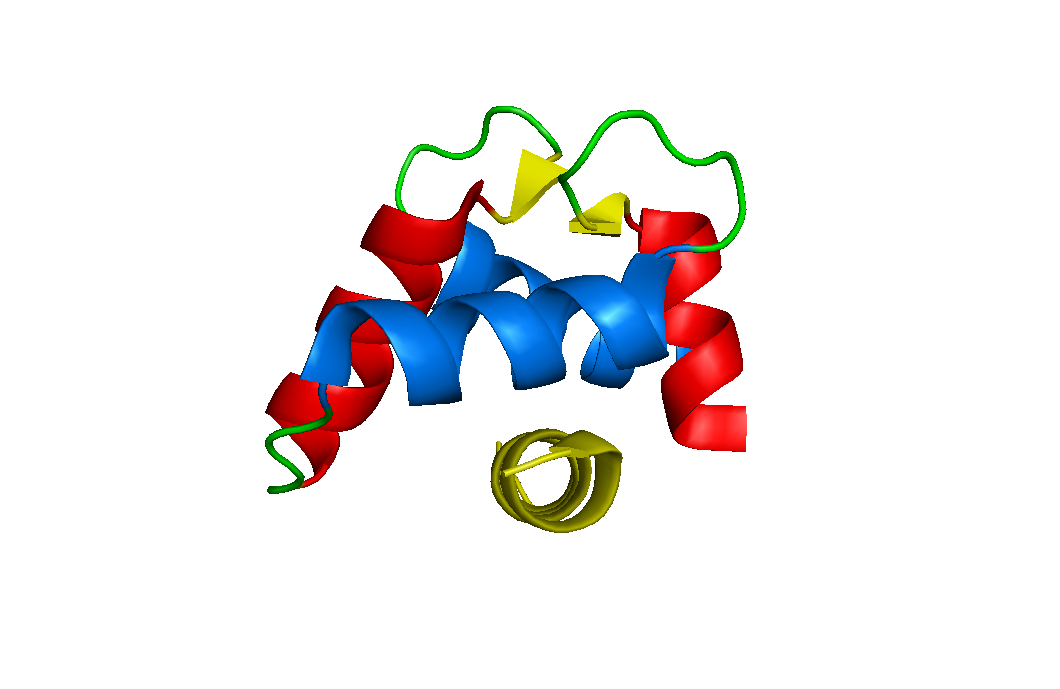
Hexa-EF-hand proteins
This subgroup contains three of the largest EF-hand family members. Calbindin D28k, Calretinin and Secretagogin all contain six EF-hand motifs. They are highly abundant in the brain and their growing absence with age correlates with Alzheimer's, Parkinson's and Huntington's. The proteins appear to act as simple calcium-buffers, mopping up the excess calcium inside the cell. However, several groups report that they interact with other proteins. Our hypothesis is that the protein interaction network depends on factors other than calcium. We want to compare the structure and interaction networks of calcium-bound calbindin and calretinin at normal and low pH. Contact Patrick (pgroves@itqb.unl.pt) about our structural program and Malgorzata (mpalczewska@itqb.unl.pt) about our search for new protein targets at low pH.
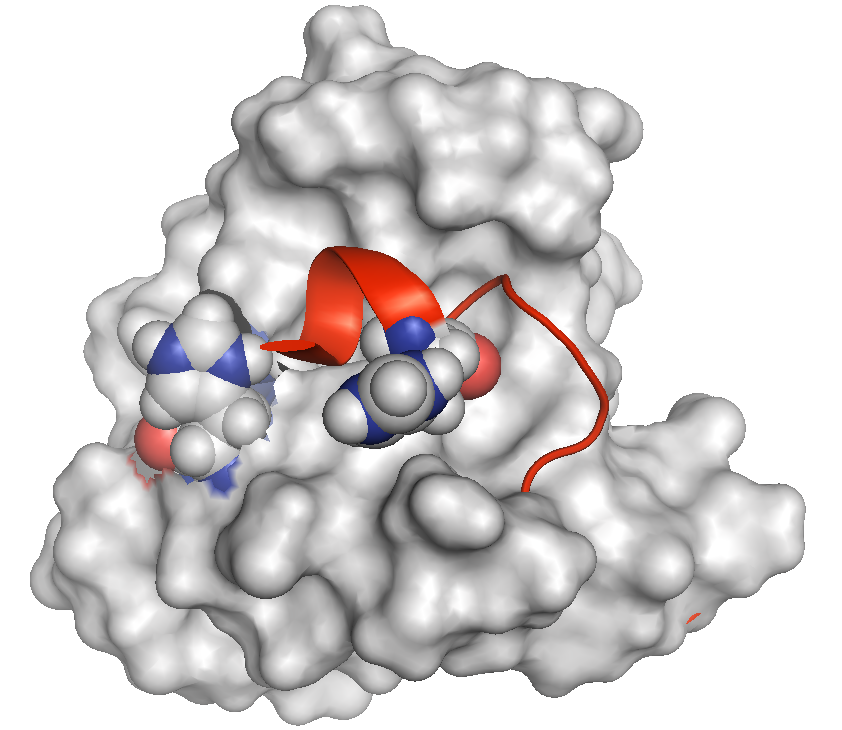
The N-terminus (red) of calbindin D28k lies across a hydrophobic cleft in calcium-bound form of the protein. The peptide occupies a space normally reserved for recognizing and binding other proteins. The protein contains two histidine residues in close proximity (shown in blue-grey CPK format) that potentially force an extrusion of the peptide at low pH when they become protonated (charged). We believe our proteins are activated (to bind other proteins) in the presence of calcium at pH 6.7, which is lower than the typical intracellular pH 7.3.
Neuronal Calcium Sensors
This subgroup, as the name implies, is also important in brain function. We are completing a project on a protein called DREAM whose function is related to its oligomeric state and localization inside the cell. This work is in collaboration with a Spanish goup.
Other EF-hand proteins
We are also interested in calmodulin, S100 proteins and new members of the family. Other EF-hand proteins provide a reference and contrast to our studies.
Annexins
Annexins are calcium-binding proteins but they are not EF-hand proteins. We work with Polish and French groups to elucidate the proteins' nucleotide-binding properties by NMR, and the role of annexins in bone formation. This project obtains some funding from a bilateral exchange. Contact Patrick (pgroves@itqb.unl.pt)
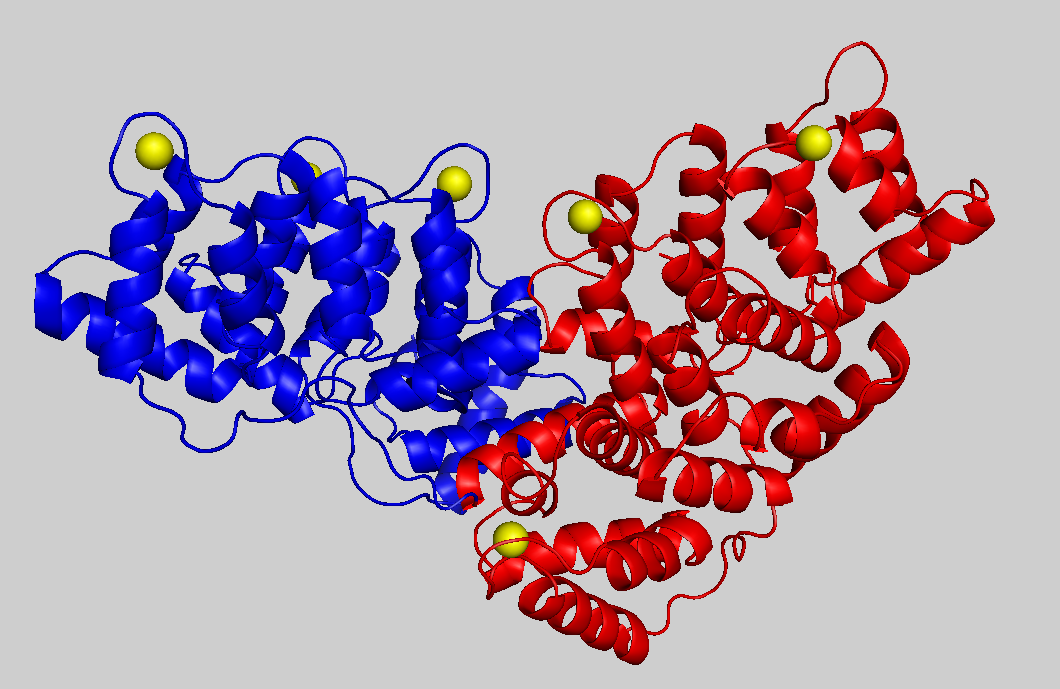
Structure of calcium-bound annexin A6. This protein is twice as large as other annexins due to gene duplication and the two subunits are painted red and blue. Five of the six calcium cations (yellow) provide the jam that sandwiches this annexin with negatively charged membranes. Nucleotides are believed to bind at the interface of the two domains and this provides the potential to alter the relative orientation of the two domains, the curvature of the protein and the curvature of the membrane surface.