[Seminar] Exploring the mechanism of metalloenzymes using microsecond timescale rapid mixing techniques
Peter-Leon Hagedoorn
| When |
22 Feb, 2023
from
02:30 pm to 03:30 pm |
|---|---|
| Where | Room 2.13 |
| Contact Name | Smilja Todorovic |
| Add event to your calendar |
|
Title: Exploring the mechanism of metalloenzymes using microsecond timescale rapid mixing techniques
Speaker: Peter-Leon Hagedoorn, Department of Biotechnology, Delft University of Technology, Delft, The Netherlands
Host: Smilja Todorovic
Abstract:
Roughly one third of all proteins contain one or more metal cofactor, and the identity, oxidation state and coordination environment of the metal cofactor provides essential information about the function of the protein. Furthermore, spectroscopy can be used to probe the electronic structure of metal cofactors while the enzyme is converting its substrate, which helps to identify catalytic intermediates and ultimately resolve a catalytic mechanisms. Enzymes are superior catalysts and can achieve turnover rates of more than a thousand per second. This makes it technically challenging to study the mechanism of such enzymes. Special ultrafast mixing techniques are necessary to solve this.
I will present the use of ultrafast mixing techniques to establish kinetic mechanisms in the case of metalloprotein folding, metalloenzyme catalysis, and metal to protein binding. Two different in-house developed ultrafast kinetic techniques were used: Nanospec, ultrafast continuous flow UV-vis spectrophotometry; MHQ, microsecond timescale rapid freeze hyperquenching. The dead times of these instruments are 100X shorter than for commercially available devices.
Cytochrome c refolding kinetics during a pH jump from 2 (unfolded) to 4.7 (near native state) was studied in the submillisecond timescale.1 The combination of the transient UV-vis and EPR data show that within 1 millisecond cytochrome c folding proceeds through three different steps: 1) His18 binding to the heme cofactor, 2) a conformational change that affects the heme environment, 3) Met80 binding to the heme cofactor in concert with a significant increase of the alpha helical content of the protein. There is evidence for further slower conformational steps towards the stable folded state at pH 4.7.
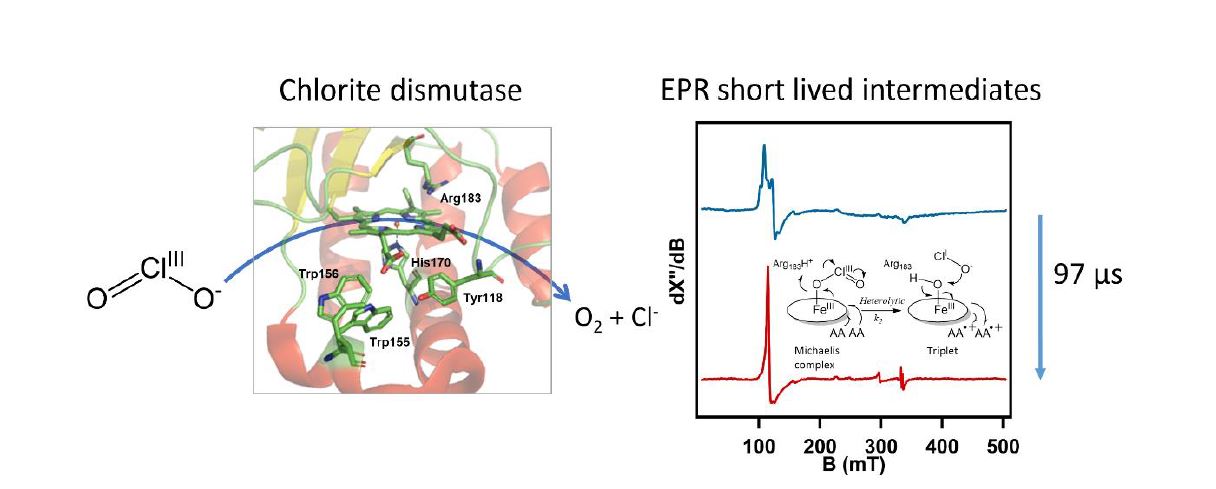
Novel intermediate states were identified in the catalytic mechanism of the fast heme enzyme chlorite dismutase.2 Chlorite dismutase is a unique heme b dependent enzyme that catalyzes the conversion of chlorite (ClO2-) to molecular oxygen (O2) and chloride (Cl-). This reaction involves O-O bond formation, which is rare in nature. The enzyme catalyzes a single turnover in less than a millisecond. The catalytic mechanism of chlorite dismutase was investigated using microsecond timescale mixing techniques and the natural substrate chlorite. The UV-visible, EPR and Resonance Raman spectra of catalytic intermediates were obtained. Distinct intermediates were found that are not observed with the artificial substrate peracetic acid. Most notably a triplet state EPR signal that we attribute to two weakly coupled amino acid based cation radicals, ‘compound T’, was transiently formed. The formation of compound T is direct evidence of a two electron transfer process which means that the Cl-O bond break is heterolytic, unlike the most recent proposed mechanism for this enzyme. To our knowledge such a triplet state has never been identified in any heme enzyme.
The kinetics of Cu2+ binding to the metal binding motifs of human high affinity copper uptake protein 1 (CTR1) showed the formation of transient binding intermediates.3 The N-terminal domain of this protein has a well-known high affinity Cu2+ binding site, but only the properties of the final complex have been studied previously. Using MHQ discrete Cu2+ binding intermediates were identified in the submillisecond to second scale. These intermediates represent the subsequent coordination to one, two and three protein ligands, clearly suggesting a binding mechanism. Furthermore some of the short lived intermediates have lifetimes and redox properties that have important physiological consequences for Cu transport and the involvement of Cu in human diseases.
- Srour, B., Strampraad, M.J.F., Hagen, W.R., Hagedoorn, P.L., Refolding kinetics of cytochrome c studied with microsecond timescale continuous-flow UV-vis spectroscopy and rapid freeze-quench EPR, J. Inorg. Biochem. 184 (2018) 42-49. https://doi.org/10.1016/j.jinorgbio.2018.04.011
- 2 Püschmann, J., Mahor, D., De Geus, D.C., Strampraad, M.J.F., Srour, B., Hagen, W.R., Todorovic, S., Hagedoorn, P.L., A unique biradical intermediate in the mechanism of the heme enzyme chlorite dismutase, ACS Catal. 11 (2021) 14533-14544. https://doi.org/10.1021/acscatal.1c03432
- 3 Kotuniak, R., Strampraad, M.J.F., Bossak-Ahmad, K., Wawrzyniak, U.E., Ufnalska, I., Hagedoorn, P.L., Bal, W., Key intermediate species reveal the Cu(II) exchange pathway in biorelevant ATCUN/NTS complexes, Angew. Chemie. Int. Ed. 59 (2020)11234-11239. https://doi.org/10.1002/ange.202004264







