There is a time for protection and a time for letting go
Balance is key for all life on Earth. All cells have several mechanisms that regulate their levels of proteins, genetic material (namely mRNAs), and other metabolites, maintaining them within certain parameters – this is called homeostasis. In the latest issue of Nature Communications, a team of ITQB NOVA scientists, in collaboration with biotech company Genentech, published a model of an unanticipated role of Pumilio, a protein of the PUM family, for the maintenance of homeostasis in the endoplasmic reticulum. PUM proteins are essential RNA Binding Proteins for development of organisms and have been associated with neurological diseases, infertility, movement disorders and cancer.
The unfolded protein response (UPR) regulates the levels of proteins in the Endoplasmic Reticulum (ER), thus avoiding their accumulation, which could be detrimental for the correct functioning of the cell. When misfolded proteins accumulate in the ER, they activate the sensor protein Ire1 (inositol-requiring enzyme 1), which then removes nucleotides (splicing) from Xbp1 (X-box binding protein 1) mRNA, through its endoribonuclease activity. Xbp1 is a transcription factor that regulates the expression of chaperones (proteins that guarantee the correct folding of other proteins) and other UPR genes.
The endoribonuclease domain of Ire1 is also responsible for regulated Ire1-dependent decay (RIDD), a degradation process of specific mRNAs or microRNAs. The activity of this domain, controlled by the phosphorylation state of Ire1, can lead to the depletion of mRNAs and affects both the ER homeostasis and cell fate. The team led by ITQB NOVA PI Pedro Domingos proposes a model for the role of Pumilio in the Ire1-Xbp1 pathway, by protecting the Xbp1 mRNA from RIDD, while maintaining its normal splicing.
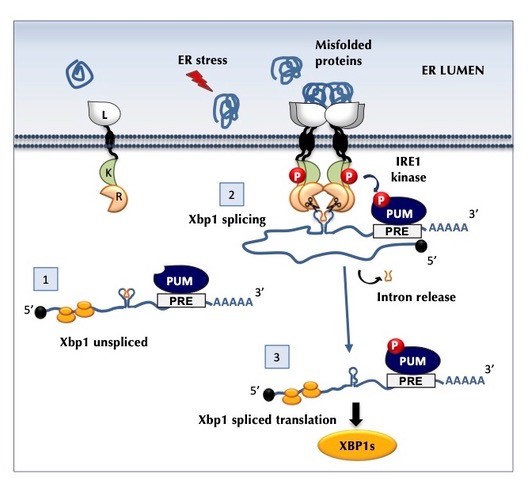
Graphical representation of the model proposed in the paper
The proposed model states that under normal conditions (no stress), the Ire1 is not active and therefore there is no splicing of Xbp1 mRNA, although the Pumilio is able to bind to the unspliced form of Xbp1 mRNA. The accumulation of misfolded proteins in the ER leads to stress and activates Ire1, which leads to the phosphorylation of Pumilio. This causes a conformational change in the interaction between Pumilio and the Xbp1 mRNA, protecting it from RIDD but maintaining it accessible for splicing by Ire1. This way, the spliced form of Xbp1 mRNA is protected and ready for translation.
Furthermore, the model predicts that when stress conditions are reduced, Pumilio phosphorylation levels will be reduced, leaving Xbp1 mRNA unprotected and subject to quality control mechanisms, including the degradation action of Ire1. This avoids the accumulation of spliced Xbp1 under non-stress conditions, which could be detrimental for cellular homeostasis.
“Although many studies have focused on the biological role of PUM proteins, little is known about their interaction and regulation of Xbp1 mRNA”, states Pedro Domingos, head of the Cell Signalling in Drosophila Lab. “This discovery furthers our understanding regarding these complex and crucial mechanisms that help maintain the equilibrium within the cell”, he adds.
“Our studies were performed mostly in Drosophila, but we used human proteins as well.”, Says Fátima Cairrão, from the same lab. “Thus, our contributions are also important to deepen our knowledge regarding the human organism and add another level of regulation on UPR mechanisms by RNA Binding Proteins.”
ITQB NOVA researchers Cristiana C. Santos (left), Fátima Cairrão (centre) and Pedro M. Domingos (right)
The research was funded by Fundação para a Ciencia e Tecnologia and ‘la Caixa’ Foundation.
Original Paper
Nature Communications | https://www.nature.com/articles/s41467-022-29105-x
Pumilio protects Xbp1 mRNA from regulated Ire1-dependent decay
Fátima Cairrão, Cristiana C. Santos, Adrien Le Thomas, Scot Marsters, Avi Ashkenazi, Pedro M. Domingos
DOI: 10.1038/s41467-022-29105-x







