To keep them out, we must learn how they got in
Parainfluenza viruses are the second biggest cause of hospitalization in children under the age of five years old with respiratory issues. Every year, millions of infants, children, fragile adults and elderly are diagnosed with diseases caused by these viruses, such as bronchiolitis and pneumonia. Currently, its treatment is merely symptomatic, given the absence of any specific prophylaxis. In the cover article of this month’s ACS Chemical Biology, a team of ITQB NOVA researchers, in collaboration with iMM, uncovered the crucial mechanisms for the entry of parainfluenza viruses into the host’s cells. This discovery can contribute to uncovering ways to inhibit entrance and prevent infection.
Parainfluenza viruses are part of the paramyxoviruses family. These enveloped viruses contain an RNA genome that requires entrance into the host cell to replicate. To allow entrance, the virus envelope must fuse with the cell membrane. The fusion process starts when a protein of the virus – glycoprotein F – recognizes and binds to the specific receptors on the cell membrane. Upon binding, glycoprotein F suffers changes in its spatial organization, exposing a region called fusion peptide. When this region is inserted into the cell membrane, it promotes membrane fusion between the virus and the host.
However, we do not yet understand a fundamental part of this process – what are the mechanisms through which the parainfluenza fusion peptide (PIFP) promotes this fusion? This ITQB NOVA and iMM paper provides answers to this question.
Researchers conclude that this peptide promotes fusion by destabilizing the host’s membrane. Upon interaction with the membrane, the peptide refolds into a helix. When enough of these peptides are inserted in the membrane, they form clusters of oligomeric structures. “These structures resemble a canal, comprised of six or nine straws, arranged in a circle that crosses the membrane of the infected cell, forming a pore through which water passes facilitating membrane fusion” explains Diana Lousa, ITQB NOVA researcher. “This is a fundamental step for the entrance of the virus’ RNA, which will then use the cell components to replicate, once inside”, adds Mariana Valério, PhD student.
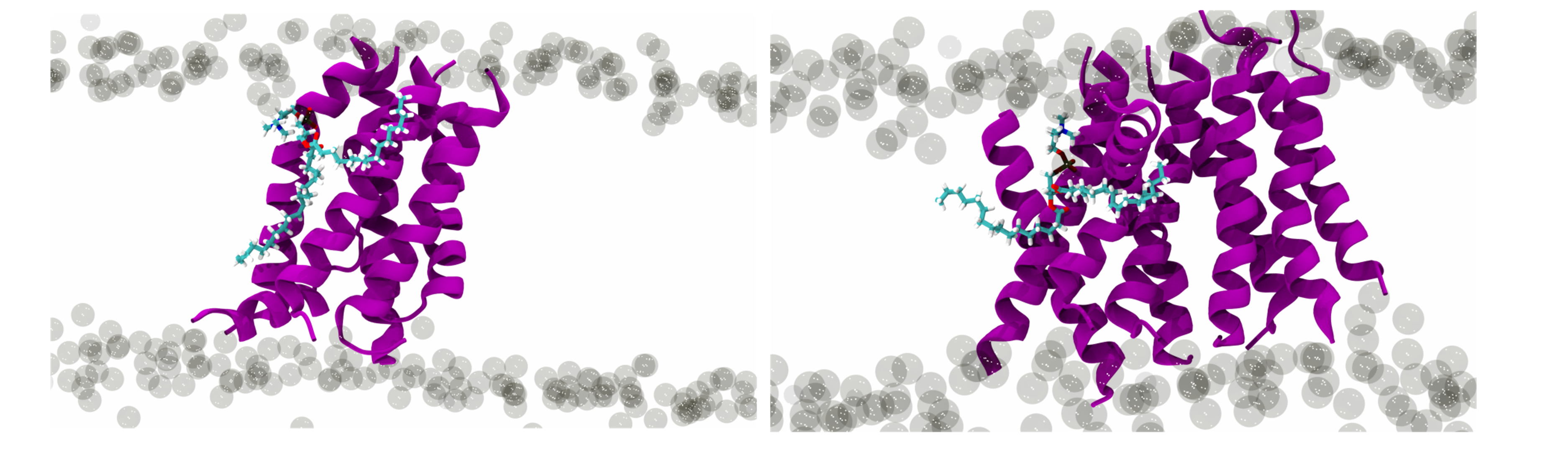
Fig. 1 - Formation of oligomeric structures of the fusion peptide in the membrane.
In spite of previous suggestions that PIFP forms these pore-like molecular structures, “it was the first time that it was demonstrated that the clustering of PIFP and the formation of these structures, that are fundamental for membrane fusion, occurs spontaneously”, elucidates Ana Salomé Veiga, iMM researcher. This collaboration between computational and experimental biology will allow for new strides in the development of antiviral therapies to help fight the spreading of diseases caused by parainfluenza viruses.
Original Paper
Parainfluenza Fusion Peptide Promotes Membrane Fusion by Assembling into Oligomeric Porelike Structures
Mariana Valério, Diogo A. Medonça, João Morais, Carolina C. Buga, Carlos H. Cruz, Miguel A. R. B. Castanho, Manuel N. Melo, Cláudia M. Soares, Ana Salomé Veiga, Diana Lousa
DOI: https://doi.org/10.1021/acschembio.2c00208







