Dissemination
Paper #19
Collaboration among @UNiVPAVIA, @UNIVGRONINGEN, @ZYMVOL and @GECCO
Broadening the Catalytic Scope of the Peroxygenase Activity of a Bacterial Tyrosine Hydroxylase
Carraretto, D, Alonso-Cotchico, Martin, C, Trajkovic, van Beek, H.L., Mattevi, Fraaije, M, Lucas, MF, Loncar, N
ChemCatChem, e202401819

December 2024
Fungal unspecific peroxygenases (UPOs, EC 1.11.2.1) are known for their unique biocatalytic properties and have been extensively studied since their discovery over two decades ago. UPOs have found various applications in various fields, such as bioremediation, drug metabolism, and synthetic organic chemistry. Nonetheless, the production of UPOs seems limited to fungal expression systems, which makes enzyme engineering challenging. It would be attractive to have a peroxygenase available that is easily expressed in a bacterial host. Here, we report on the expression and engineering of a bacterial peroxygenase, tyrosine hydroxylase (TyrH, EC 1.11.2.6). With only two rounds of computer-aided engineering, we have identified distal and active-site mutations that improve enzymatic activities and substrate scope, respectively. Combining these mutations in a single variant led to an effective peroxygenase capable of indigo production and enantioselective sulfoxidation, which can be overexpressed in Escherichia coli.
Paper #18
Collaboration between @CONICET and @ITQB
Scocozza, MF, Zitare, UA, Cancian, P, Castro, MA, Martins, LO, Murgida, DH.
J. Inorg. Biochem. 2024

December 2024
In this paper, it is shown that replacing the distal residues Asp and/or Arg of the DyP peroxidases from Bacillus subtilis and Pseudomonas putida results in functional enzymes, albeit with spectroscopically perturbed active sites. All the enzymes can be activated by adding exogenous H2O2 or by in situ electrochemical generation of the reactive oxygen species (ROS) •OH, O2•- and H2O2. The latter method leads to broader and upshifted pH-activity profiles. Both WT enzymes exhibit a differential predominance of ROS involved in their electrochemical activation, which follows the order •OH > O2•- > H2O2 for BsDyP and O2•- > H2O2 > •OH for PpDyP. This ROS selectivity is preserved in mutants with unperturbed sites but is blurred out for distorted sites. The underlying molecular basis of the selectivity mechanisms is analyzed through molecular dynamics simulations, which reveal distorted hydrogen bonding networks and higher throughput of the access tunnels in the variants exhibiting no selectivity. The electrochemical activation method provides superior performance for protein variants with a high prevalence of the alternative •OH and O2•- species. These results constitute a promising advance towards engineering DyPs for electrocatalytic applications.
Paper #17
Collaboration between @CONICET and @RUG
ALACEN: A Holistic Herbaceous Biomass Fractionation Process Attaining a Xylose-Rich Stream for Direct Microbial Conversion to Bioplastics
Salvador Bertran-Llorens, Wen Zhou, Martín A. Palazzolo, Dana l. Colpa, Gert-Jan W. Euverink, Janneke Krooneman and Peter J. Deuss
ACS Sustainable Chemistry & Engineering 2024, https://doi.org/10.1021/acssuschemeng.3c08414
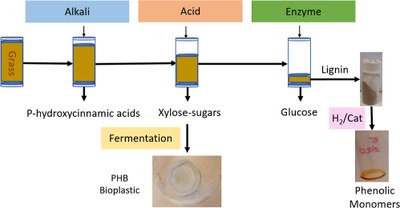
May 2024
Lignocellulose biorefining is a promising technology for the sustainable production of chemicals and biopolymers. Usually, when one component is focused on, the chemical nature and yield of the others are compromised. Thus, one of the bottlenecks in biomass biorefining is harnessing the maximum value from all of the lignocellulosic components. Here, we describe a mild stepwise process in a flow-through setup leading to separate flow-out streams containing cinnamic acid derivatives, glucose, xylose, and lignin as the main components from different herbaceous sources. The proposed process shows minimal degradation of the individual components, and conservation of their natural structure is possible. Under optimized conditions, the following fractions are produced from wheat straw based on their respective contents in the feed by the ALkaline ACid ENzyme process: (i) 78% ferulic acid from a mild ALkali step, (ii) 51% monomeric xylose free of fermentation inhibitors by mild ACidic treatment, (iii) 82% glucose from ENzymatic degradation of cellulose, and (iv) 55% native-like lignin. The benefits of using the flow-through setup are demonstrated. HSQC NMR confirmed the retention of the lignin aryl ether structureR, and this allowed monomers to form from hydrogenolysis. More importantly, the crude xylose-rich fraction was shown to be suitable for producing polyhydroxybutyrate bioplastics. The direct use of the xylose-rich fractionating the thermophilic bacteria Schlegelella thermodepolymerans matched 91% of the PHA produced with commercial pure xylose, achieving 138.6 mgPHA/xylose. Overall, the ALACEN fractionation method allows for a holistic valorization of the principal components of herbaceous biomasses.
Paper #16
Collaboration among @ITQB-NOVA and @GECCO
Spectroelectrochemistry for determination of the redox potential in heme enzymes: Dye-decolorizing peroxidases
Catarina Barbosa, Carolina F. Rodrigues, Nikola Lončar, Lígia O. Martins, Smilja Todorovic & Célia M. Silveira
BBA Advances 2024 https://doi.org/10.1016/j.bbadva.2023.100112
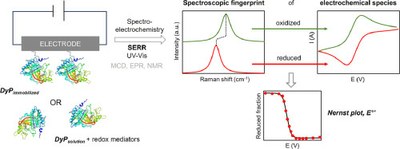
April 2024
Dye-decolorizing peroxidases (DyPs) are heme-containing enzymes that are structurally unrelated to other peroxidases. Some DyPs show high potential for applications in biotechnology, which critically depends on the stability and redox potential (E°') of the enzyme. Here we provide a comparative analysis of UV–Vis- and surface-enhanced resonance Raman-based spectroelectrochemical methods for determination of the E°' of DyPs from two different organisms, and their variants generated targeting E°' upshift. We show that substituting the highly conserved Arginine in the distal side of the heme pocket by hydrophobic amino acid residues impacts the heme architecture and redox potential of DyPs from the two organisms in a very distinct manner. We demonstrate the advantages and drawbacks of the used spectroelectrochemical approaches, which is relevant for other heme proteins that contain multiple heme centers or spin populations.
Paper #15
Collaboration among @ITQB-NOVA, @CONICET
Superoxide versus peroxide activation of dye decolorizing peroxidases for bioelectrocatalysis
Ulises A. Zitare, Francisco Vieyra, Magalí F. Scocozza, Francisco Rosciani, María A. Castro, Ligia O. Martins & Daniel H. Murgida
Bioresource Technology Reports 2024 https://doi.org/10.1016/j.biteb.2024.101819
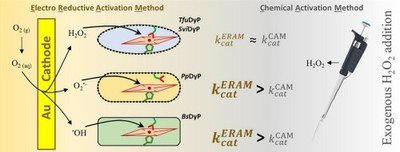
March 2024
This work shows thatdye-decolorizingg peroxidases can be electrochemically activated for substrate oxidation without the addition of exogenous H2O2, yielding activities similarto or better than in their conventional operation. The method is based on the in situ generation of O2•−, •OH and H2O2. These species bind the heme to form catalytically competent oxoiron intermediates. Activation of the Pseudomonas putida enzyme is mainly mediated by O2•−, while those from Thermobifida fusca and Saccharomonospora viridis show preferential activation by H2O2. This differential reactivity is explained by the dynamics of a conserved distal aspartate residue. In contrast to previous reports on the Bacillus subtilisenzyme, none of the peroxidases studied here show significant •OH mediated activation, as this radical is quenched by reactive amino acids abundant in these proteins. These results pave the way for the development of bioelectrocatalytic reactors based on dye decolorizing peroxidases for water remediation and biomass processing.
Paper #14
Collaboration among @ITQB-NOVA, @Zymvol Biomodeling
Flexible active-site loops fine-tune substrate specificity of hyperthermophilic metallo-oxidases
Vânia Brissos, Patrícia T. Borges, Ferran Sancho, Maria Fátima Lucas, Carlos Frazão, Felipe Conzuelo & Lígia O. Martins
Journal of Biological Inorganic Chemistry 2024 https://doi.org/10.1007/s00775-023-02040-y
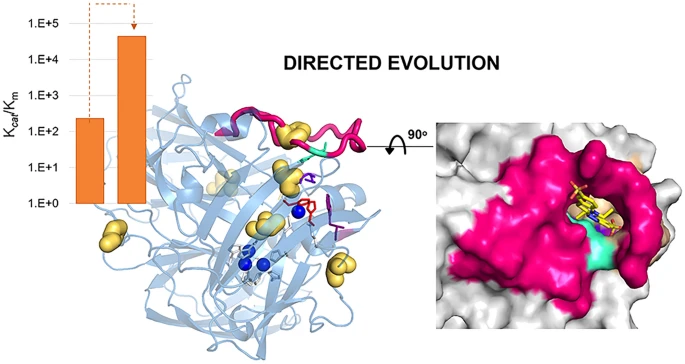
January 2024
Hyperthermophilic (‘superheat-loving’) archaea found in high-temperature environments such as Pyrobaculum aerophilum contain multicopper oxidases (MCOs) with remarkable efficiency for oxidizing cuprous and ferrous ions. In this work, directed evolution was used to expand the substrate specificity of P. aerophilum McoP for organic substrates. Six rounds of error-prone PCR and DNA shuffling followed by high-throughput screening lead to the identification of a hit variant with a 220-fold increased efficiency (kcat/Km) than the wild-type for 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) without compromising its intrinsic activity for metal ions. TheX-ray crystal structure analysise reveals four proximal mutations close to the T1Cu active site. One of these mutations is within the 23-residues loop that occludes this site, a distinctive feature of prokaryotic MCOs. The increased flexibility of this loop results in an enlarged tunnel and one additional pocket that facilitates bulky substrate-enzyme interactions. These findings underscore the synergy between mutations that modulate the dynamics of the active-site loop enabling enhanced catalytic function. This study highlights the potential of targeting loops close to the T1Cu for engineering improvements suitable for biotechnological applications.
Paper #13
Collaboration among @ITQB-NOVA, @Zymvol Biomodeling
Mechanistic insights into glycoside 3-oxidases involved in C-glycoside metabolism in soil microorganisms
André Taborda, Tomás Frazão, Miguel V. Rodrigues, Xavier Fernández-Luengo, Ferran Sancho, Maria Fátima Lucas, Carlos Frazão, Eduardo P. Melo, M. Rita Ventura, Laura Masgrau, Patrícia T. Borges & Lígia O. Martins
Nature Communications 2023 https://doi.org/10.1038/s41467-023-42000-3
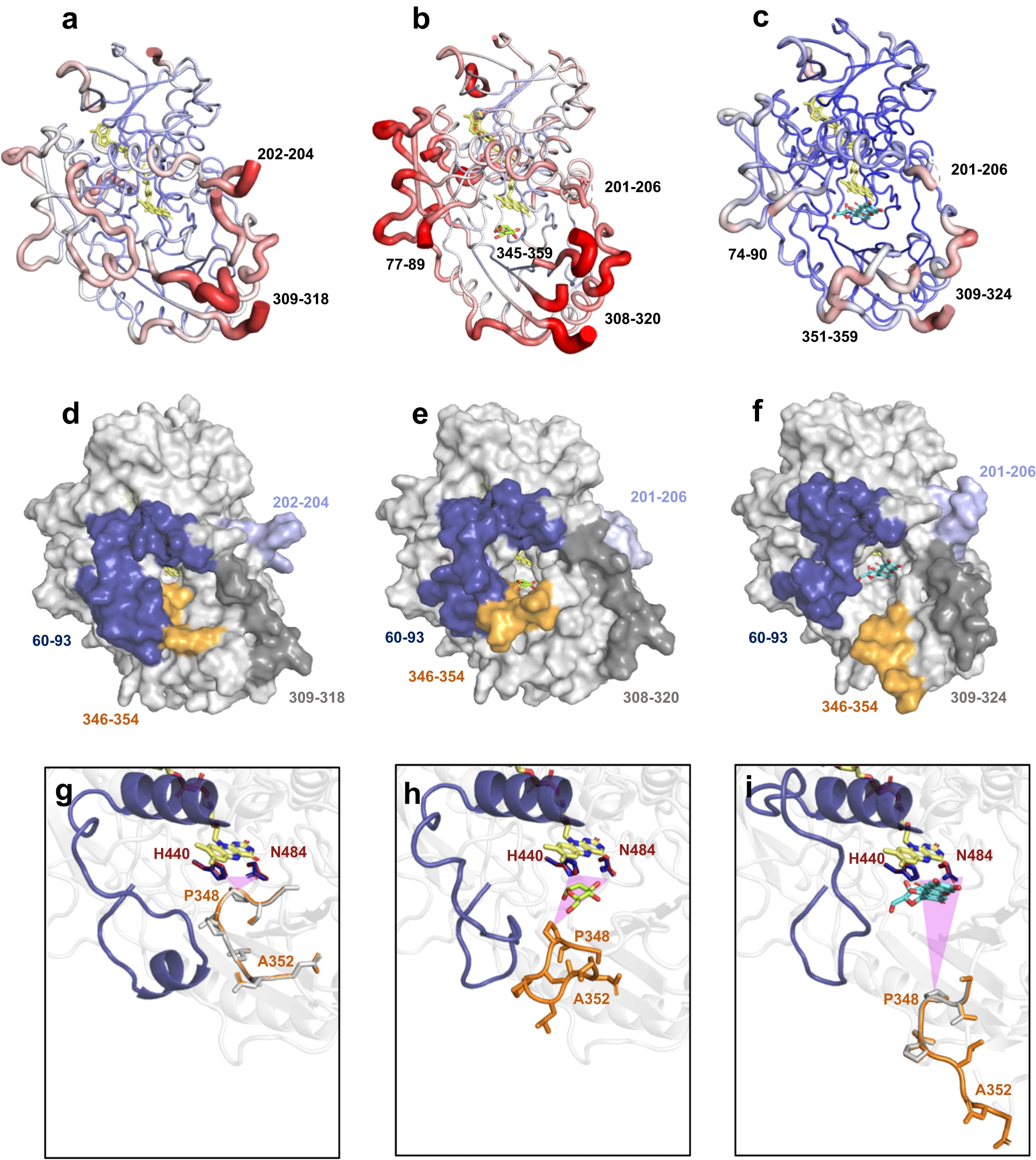
November 2023
C-glycosides are natural products with important biological activities but are recalcitrant to degradation. Glycoside 3-oxidases (G3Oxs) are recently identi- fied bacterial flavo-oxidases from the glucose-methanol-coline (GMC) super- family that catalyze the oxidation of C-glycosides with the concomitant reduction of O2 to H2O2. This oxidation is followed by C-C acid/base-assisted bond cleavage in two-step C-deglycosylation pathways. Soil and gut micro- organisms have different oxidative enzymes, but the details of their catalytic mechanisms are largely unknown. Here, we report that PsG3Ox oxidizes at 50,000-fold higher specificity (kcat/Km) the glucose moiety of mangiferin to 3- keto-mangiferin than free D-glucose to 2-keto-glucose. Analysis of PsG3Ox X-ray crystal structures and PsG3Ox in complex with glucose and mangiferin, combined with mutagenesis and molecular dynamics simulations, reveal dis- tinctive features in the topology surrounding the active site that favor cata- lytically competent conformational states suitable for recognition, stabilization, and oxidation of the glucose moiety of mangiferin. Furthermore, their distinction to pyranose 2-oxidases (P2Oxs) involved in wood decay and recycling is discussed from an evolutionary, structural, and functional viewpoint.
Paper #12
Collaboration among @RUG, @Zymvol Biomodeling & @UniPavia
One-Pot Biocatalytic Synthesis of rac-Syringaresinol from a Lignin-Derived Phenol
Yiming Guo, Laura Alvigini, Mohammad Saifuddin, Ben Ashley, Milos Trajkovic, Lur Alonso-Cotchico, Andrea Mattevi, and~Marco W. Fraaije
ACS Catalysis 2023 https://doi.org/10.1021/acscatal.3c04399
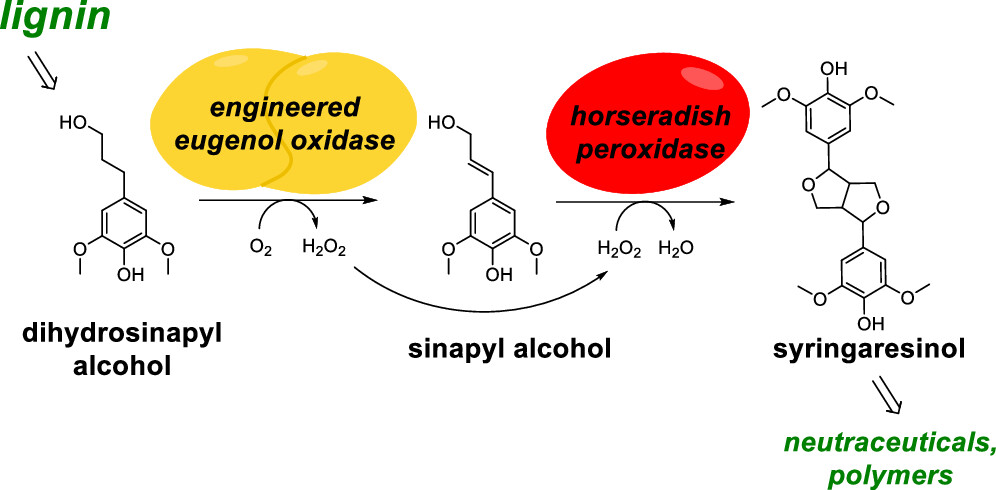
November 2023
We present a one-pot biocatalytic cascade reaction for producing racemic syringaresinol, a lignan with applications as a nutraceutical and in polymer chemistry. The process consumes dihydrosinapyl alcohol, which can be produced renewably from the lignocellulosic material. To achieve this, a variant of eugenol oxidase was engineered to oxidize dihydrosinapyl alcohol into sinapyl alcohol with good conversion and chemoselectivity. The crystal structure of the engineered oxidase revealed the molecular basis of the influence of the mutations on the chemoselectivity of the oxidation of dihydrosinapyl alcohol. By using horseradish peroxidase, the subsequent oxidative dimerization of sinapyl alcohol into syringaresinol was achieved. Conditions for the one-pot, two-enzyme synthesis were optimized, and a high yield of syringaresinol was achieved by cascading the oxidase and peroxidase steps stepwise. This study demonstrates the efficient production of syringaresinol from a compound that can be renewed by reductive catalytic fractionation of lignocellulose, providing a biocatalytic route for generating a valuable compound from lignin.
Paper #11
Collaboration among @ITQB and @CONICET
Electrochemical Actuation of a DyP Peroxidase: A Facile Method for Drastic Improvement of the Catalytic Performance
Magalí F. Scocozza, Francisco Vieyra, Fernando Battaglini, Ligia O. Martins, and Daniel H. Murgida
ACS Catalysis 2023 https://doi.org/10.1021/acscatal.3c01530
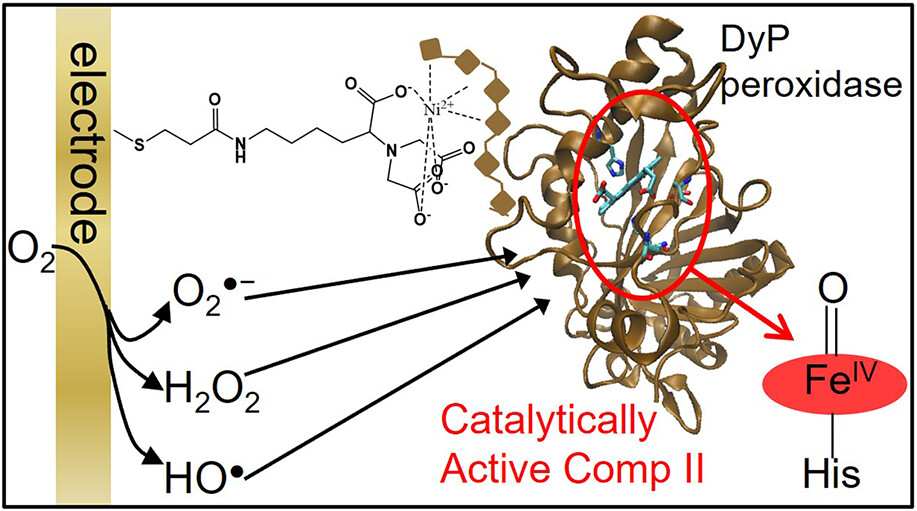
May 2023
Dye decolorizing peroxidases (DyP) have attracted interest for applications such as dye-containing wastewater remediation and biomass processing. So far, efforts to improve operational pH ranges, activities, and stabilities have focused on site-directed mutagenesis and directed evolution strategies. Here, we show that the performance of the DyP from Bacillus subtilis can be drastically boosted without the need for complex molecular biology procedures by simply activating the enzyme electrochemically in the absence of externally added H2O2. Under these conditions, the enzyme shows specific activities toward various chemically different substrates that are significantly higher than in its canonical operation. Moreover, it presents much broader pH activity profiles with the maxima shifted from neutral to alkaline. We also show that the enzyme can be successfully immobilized on biocompatible electrodes. When actuated electrochemically, the enzymatic electrodes have two orders of magnitude higher turnover numbers than with the standard H2O2-dependent operation and preserve about 30% of the initial electrocatalytic activity after 5 days of operation−storage cycles.
Dissemination in the BioTrans 2023 meeting in La Rochelle, France
25-29th June 2023
https://www.biotrans2023.com/
B-LigZymes Ph.D. Students and Post-Docs presented their incredible work on BioTrans2023. Anett Schallmey from Technische Universität Braunschweig also had a fantastic presentation on loop engineering for improved enzyme activity and enantioselectivity.

Claudia Crestini and Nicolò Pajer (Ca´Foscaria, UniVE, Italia) have participated in the Gordon Research Conference "Realizing Lignin's Potential in Biorefining by Bridging Plant Biology, Chemistry and Engineering"
1-5th August 2022
The goal of the Lignin GRC is to bring together researchers from academia, government research centers, and industry working on all aspects of lignin, including its characterization, planta engineering, utilization, depolymerization, and upgrading. Interaction, discussion, and teamwork among plant researchers, microbiologists, chemists, engineers, and material scientists will be critical to moving the field forward toward harnessing this abundant, renewable, yet incredibly challenging resource.

Magalí Scocozza (INQUIMAE) was awarded the BEST ORAL PRESENTATION at the Oxizymes2022 meeting!! ![]()
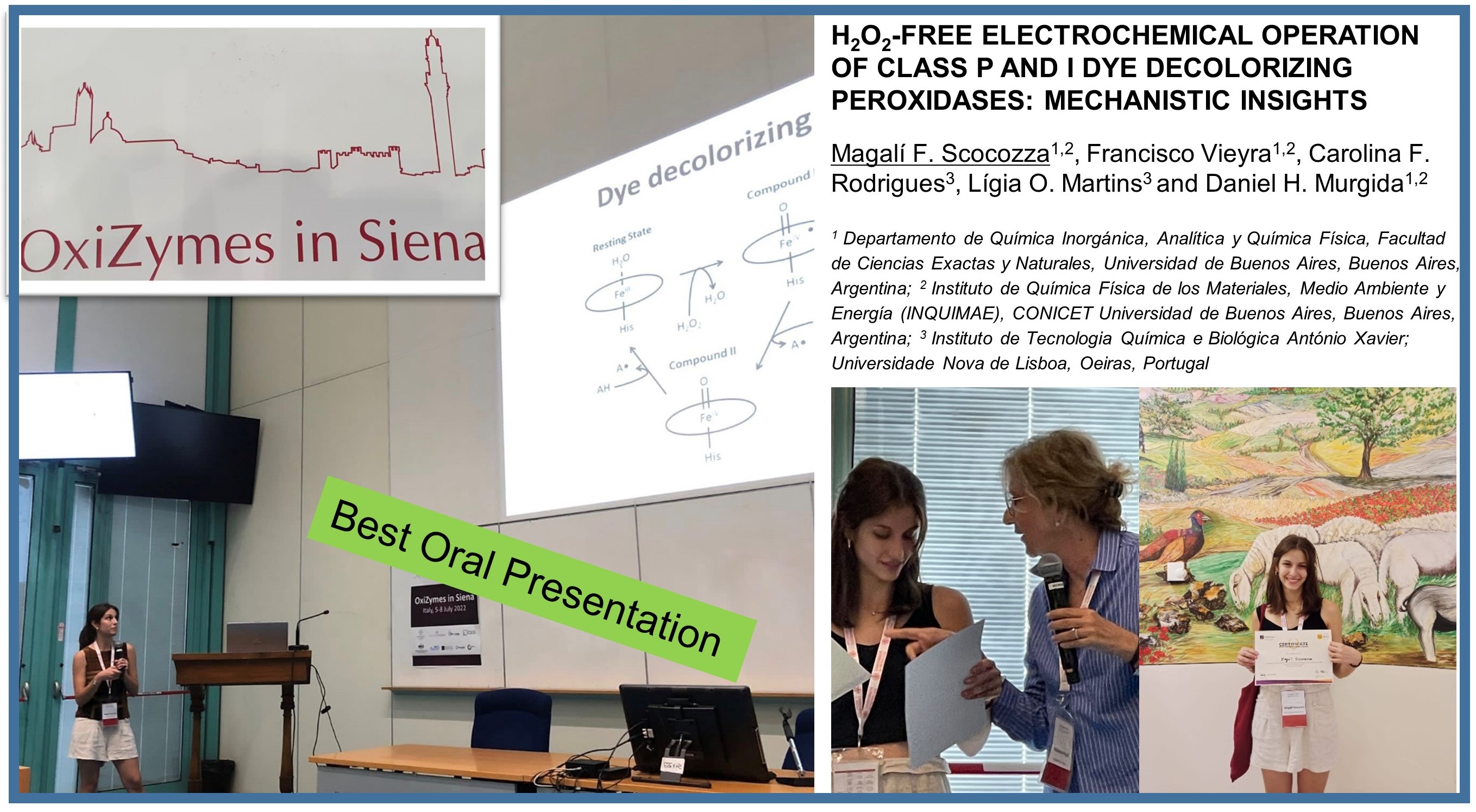
Dissemination in the Oxizymes 2022 meeting in Siena, Italy
5-8th July 2022
http://www.congressi.unisi.it/oxizymes22/
OxiZymes 2022 emphasizes the discovery of new oxidoreductases and the latest advances in their biotechnological applications. The conference provides a unique opportunity for industrial researchers to meet with foremost young academics for knowledge transfer and to establish research collaborations. The conference format is designed to be highly interactive, with ample time for stimulating discussions, interdisciplinary interactions, and meetings alongside the scientific sessions.
During the 2022 edition of Oxyzimes, B-LigZymes coordinator Lígia Martins (ITQB), partners Marco Frailje (RUG), and Lur Alonso (ZYMVOL), and Smilja Todorovic (ITQB), and Secondee Magali Scocozza (INQUIMAE), presented oral communications of their work and recent achievements.
18 posters, from B-LigZymes, associated Post-Doc and Ph.D. students, as well as GECCO CEO Nikola Loncar.
The B-LigZymes RISE Projected was represented by 8 partners, including ITQB, RUG, INQUIMAE, INTEQUI, UniPV, ZymVol, METGEN, and Gecco.

Paper #10
Collaboration among @ITQB-UNL, @Zymvol Biomodeling & UniPavia
Rationally Guided Improvement of NOV1 Dioxygenase for the Conversion of Lignin-Derived Isoeugenol to Vanillin
Mario De Simone, Laura Alvigni, Lur Alonso-Cotchico, Vânia Brissos, Jonatan Caroli, Maria Fátima Lucas, Emanuele Monza, Eduardo Pinho e Melo, Andrea Mattevi, Lígia O Martins
Biochemistry 2022 https://doi.org/10.1021/acs.biochem.2c00168
June 2022
This study integrates computational, kinetic, structural, and biophysical approaches to characterize a new NOV1 variant featuring improved activity and stability compared to those of the wild type. The S283F replacement results in a 2-fold increased turnover rate (kcat) for isoeugenol and a 4-fold higher catalytic efficiency (kcat/Km) for molecular oxygen compared to those of the wild type. Furthermore, the variant exhibits a half-life that is 20-fold higher than that of the wild type, which most likely relates to the enhanced stabilization of the iron cofactor in the active site. Molecular dynamics supports this view, revealing that the S283F replacement decreases the optimal pKa and favors conformations of the iron-coordinating histidines compatible with an increased level of binding to iron. Importantly, whole cells containing the S283F variant catalyze the conversion of ≤100 mM isoeugenol to vanillin, yielding >99% molar conversion yields within 24 h. This integrative strategy provided a new enzyme for biotechnological applications and mechanistic insights that will facilitate the future design of robust and efficient biocatalysts.
Paper #9
Collaboration between @ITQB-UNL and @Zymvol Biomodeling
Distal Mutations Shape Substrate-Binding Sites during Evolution of a Metallo-Oxidase into a Laccase
Vânia Brissos, Patrícia T. Borges, Reyes Núñez-Franco, Maria Fátima Lucas, Carlos Frazão, Emanuele Monza, Laura Masgrau,Tiago N. Cordeiro, and Lígia O. Martins
ACS Catalysis 2022, https://doi.org/10.1021/acscatal.2c00336
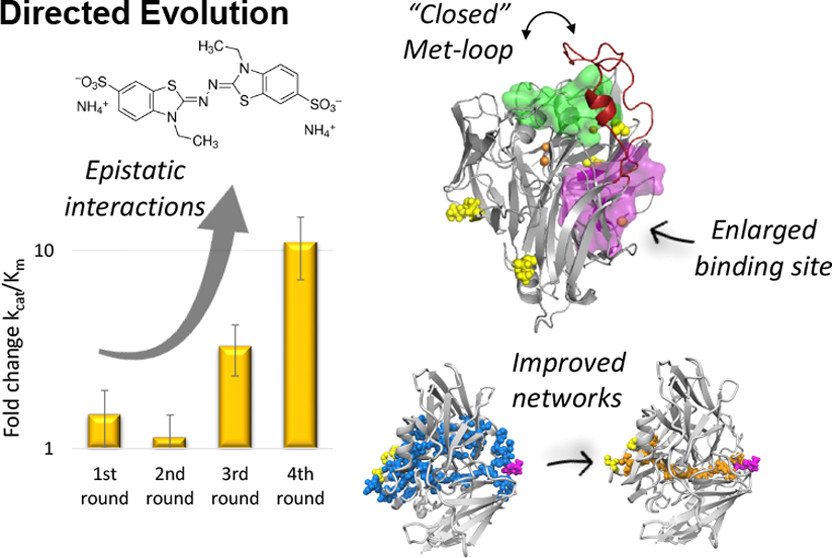
April 2022
Laccases are in increasing demand as innovative solutions in the biorefinery fields. Here, we combine mutagenesis with structural, kinetic, and in silico analyses to characterize the molecular features that cause the evolution of a hyperthermostable metallo-oxidase from the multicopper oxidase family into a laccase (kcat 273 s–1 for a bulky aromatic substrate). We show that six mutations scattered across the enzyme collectively modulate dynamics to improve the binding and catalysis of a bulky aromatic substrate. The replacement of residues during the early stages of evolution is a stepping stone for altering the shape and size of substrate-binding sites. Binding sites are then fine-tuned through high-order epistasis interactions by inserting distal mutations during later stages of evolution. Allosterically coupled, long-range dynamic networks favor catalytically competent conformational states that are more suitable for recognizing and stabilizing the aromatic substrate. This work provides mechanistic insight into enzymatic and evolutionary molecular mechanisms and spots the importance of iterative experimental and computational analyses to understand local-to-global changes.
Paper #8
Collaboration between @ITQB-UNL and @INQUIMAE-CONICET
Magalí F Scocozza, Lígia O Martins, Daniel Murgida
Int J Mol Sci 2021, https://doi.org/10.3390/ijms222212532
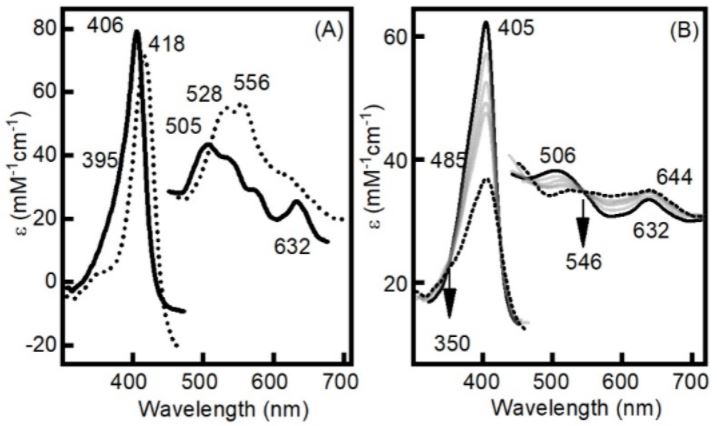
November 2021
This work introduces a novel way to obtain catalytically competent oxyferryl species for two different dye-decolorizing peroxidases (DyPs) in the absence of H2O2 or any other peroxide by simply applying a reductive electrochemical potential under aerobic conditions. UV-vis and resonance Raman spectroscopies show that this method yields long-lived compounds II and I for the DyPs from Bacillus subtilis (BsDyP; Class I) and Pseudomonas putida (PpDyP; Class P), respectively. Both electrochemically generated high valent intermediates are able to oxidize ABTS at both acidic and alkaline pH. Interestingly, the electrocatalytic efficiencies obtained at pH 7.6 are very similar to the values recorded for regular catalytic ABTS/H2O2 assays at the optimal pH of the enzymes, ca. 3.7. These findings pave the way for the design of DyP-based electrocatalytic reactors operable in an extended pH range without the need for harmful reagents such as H2O2.
Paper #7
Collaboration between @ITQB-UNL and @INQUIMAE-CONICET
Carolina F Rodrigues, Patrícia T Borges, Magalí F Scocozza, Diogo Silva, André Taborda, Vânia Brissos, Carlos Frazão, Lígia O Martins
Int J Mol Sci 2021, https://doi.org/10.3390/ijms221910862
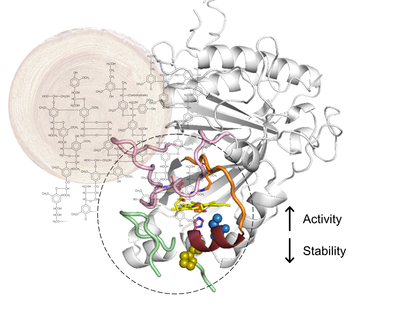
October 2021
This work reports the optimization of BsDyP from Bacillus subtillis using directed evolution for improved oxidation of 2,6-dimethoxyphenol, a model lignin-derived phenolic. BsDyP belongs to class I of the dye-decolorizing peroxidase (DyP) family of enzymes and is an interesting biocatalyst due to its high redox potential, broad substrate spectrum, and thermostability. After three rounds of evolution, one variant was identified displaying 7-fold higher catalytic rates and higher production yields as compared to the wild-type enzyme. The analysis of X-ray structures of the wild type and the evolved variant showed that the heme pocket is delimited by three long conserved loop regions and a small α helix where, incidentally, the mutations were inserted in the course of evolution. One loop in the proximal side of the heme pocket becomes more flexible in the evolved variant and the size of the active site cavity is increased, as well as the width of its mouth, resulting in an enhanced exposure of the heme to solvent. These conformational changes have a positive functional role in facilitating electron transfer from the substrate to the enzyme. However, they concomitantly resulted in decreasing the enzyme’s overall stability by 2 kcal/mol, indicating a trade-off between functionality and stability. Furthermore, the evolved variant exhibited slightly reduced thermal stability compared to the wild type. The obtained data indicate that understanding the role of loops close to the heme pocket in the catalysis and stability of DyPs is critical for the development of new and more powerful biocatalysts: loops can be modulated for tuning important DyP properties such as activity, specificity, and stability.
Paper #6
Collaboration between @ ITQB-UNL, @GECCO, and @RUG
SERR Spectroelectrochemistry as a Guide for Rational Design of DyP-Based Bioelectronics Devices
Lidia Zuccarello, Catarina Barbosa, Edilson Galdino, Nikola Loncar, Célia M. Silveira, Marco W. Fraaije and Smilja Todorovic
Int. J. Mol. Sci. 2021, https://doi.org/10.3390/ijms22157998
July 2021
Immobilized dye-decolorizing peroxidases (DyPs) are promising biocatalysts for the development of biotechnological devices such as biosensors for the detection of H2O2. To this end, these enzymes have to preserve native, solution properties upon immobilization on the electrode surface. In this work, DyPs from Cellulomonas bogoriensis (CboDyP), Streptomyces coelicolor (ScoDyP), and Thermofibida fusca (TfuDyP) are immobilized on biocompatible silver electrodes which were functionalized with alkanethiols. Their structural, redox and catalytic properties upon immobilization are evaluated by surface-enhanced resonance Raman (SERR) spectroelectrochemistry and cyclic voltammetry. Among the studied electrode/DyP constructs, only CboDyP shows preserved native structure upon attachment to the electrode. However, a comparison of the redox potentials of the enzyme in solution and immobilized states reveals a large discrepancy, and the enzyme shows no electrocatalytic activity in the presence of H2O2. While some immobilized DyPs outperform existing peroxidase-based biosensors, others fail to fulfill the essential requirements that guarantee their applicability in the immobilized state. The capacity of SERR spectroelectrochemistry for fast screening of the performance of immobilized heme enzymes places it in the front-line of experimental approaches that can advance the search for promising DyP candidates.
Paper #5
Collaboration between @INQUIMAE-CONICET, @ ITQB-UNL and @RUG
Mutational and structural analysis of an ancestral fungal dye decolorizing peroxidase
Ulises A. Zitare, Mohamed H. Habib, Henriette Rozeboom, Maria L. Mascotti, Smilja Todorovic, Marco W. Fraaije
FEBS J 2020, https://doi.org/10.1111/febs.15687
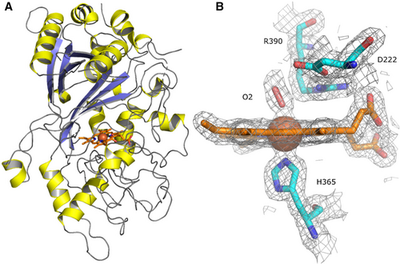
January 2021
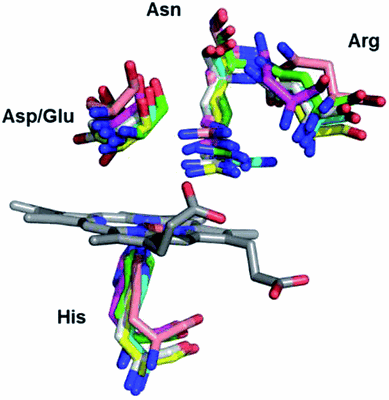
Dye decolorizing peroxidases (DyPs) constitute a superfamily of heme‐containing peroxidases that are related neither to animal nor to plant peroxidase families. These are divided into four classes (type A, B, C, and D) based on sequence features. The active site of DyPs contains two highly conserved distal ligands, an aspartate, and arginine, the roles of which are still controversial. These ligands have mainly been studied in class A‐C bacterial DyPs, largely because no effective recombinant expression systems have been developed for the fungal (D‐type) DyPs. In this work, we employ ancestral sequence reconstruction (ASR) to resurrect a D‐type DyP ancestor, AncDyPD‐b1. Expression of AncDyPD‐b1 in Escherichia coli results in large amounts of a heme‐containing soluble protein and allows for the first mutagenesis study on the two distal ligands of a fungal DyP. UV‐Vis and resonance Raman (RR) spectroscopic analyses, in combination with steady-state kinetics and the crystal structure, reveal fine pH-dependent details about the heme active site structure and show that both the aspartate (D222) and the arginine (R390) are crucial for hydrogen peroxide reduction. Moreover, the data indicate that these two residues play important but mechanistically different roles in the intra‐protein long‐range electron transfer process.
B-LigZymes sponsorships the Biocatalysis Open Day 2020
26th November 2020
The Biocatalysis Division of the European Federation of Biotechnology is a network of researchers from European universities, research institutions, and industry. Members are linked by their interest in biocatalysis and its application to solve current problems of Society in different industrial sectors including energy, chemistry, food, and biomedicine. The research areas cover a wide spectrum of topics from discovering new reactions to creating new diagnosis methods to revalorizing by-products from industry and promoting a circular and less contaminating economy.
The inaugural meeting of the EFB Biocatalysis Division will be an Open Day on the 26th of November 2020. It will be an online event consisting of plenary lectures, short invited lectures, and flash talks focusing on diverse aspects of biocatalysis.
B-Ligzymes sponsors the Biocatalysis Open day and several B-LigZymes researchers will be presenting their work there. Our Coordinator Professor Lígia O. Martins is the chair of the first session. Have a look at the program and stay tuned for more events here.
Contribution to the book “Laccase in Bioremediation and Waste Valorization” Springer, edited by Dietmar Schlosser.
Chapter 2 "Bacterial Laccases: Some Recent Advances and Applications"
Martins L.O., Melo E.P., Sanchez-Amat A., Robalo M.P.
Microbiology Monographs, vol 33. Springer, Cham.

August 2020
Laccases belong to the large family of multi-copper oxidases (MCOs) that couple the one-electron oxidation of substrates with the four-electron reduction of molecular oxygen to water. Because of their high relative non-specific oxidation capacity particularly on phenols and aromatic amines as well as the lack of requirement for expensive organic cofactors, they have found application in a large number of biotechnological fields. The vast majority of studies and applications were performed using fungal laccases, but bacterial laccases show interesting properties such as optimal temperature above 50 °C, optimal pH at the neutral to alkaline range, thermal and chemical stability, and increased salt tolerance. Additionally, bacterial systems benefit from a wide range of molecular biology tools that facilitates their engineering and achievement of high yields of protein production and setup of cost-effective bioprocesses. In this review, we provided up-to-date information on the distribution and putative physiological role of bacterial laccases and highlight their distinctive structural and biochemical properties, discuss the key role of copper in the biochemical properties, discuss thermostability determinants and, finally, review biotechnological applications with a focus on catalytic mechanisms on phenolics and aromatic amines.
Online Workshop on "Innovative Solutions to Valorize Lignin" has the participation of our partner GECCO's CEO Nikola Lončar
30th June 2020
This workshop was organized by Cambridge Nanomaterials Technology Ltd and LEITAT Technological Center. Nicola talked about biocatalytic production of lignin oligomers and the use of enzymes in bio-refinery and research and development done on ligninolytic enzymes within the B-LigZymes RISE European Commission Project.
This event presented innovative solutions at various technological readiness levels that valorize lignin coming from the paper industry. Participants learn what are the objectives in Europe regarding lignin valorization, problems faced by the paper industry, solutions implemented during lignin production, and finally different technologies available to valorize lignin. Speakers were from small and large industries but also research centers that will present results of development activities.
Have a look at the agenda:

Paper #4
Collaboration between @ ITQB-UNL and @Zymvol Biomodeling
Patrícia T. Borges, Vânia Brissos, Guillem Hernandez, Laura Masgrau, Maria Fátima Lucas, Emanuele Monza, Carlos Frazão, Tiago N. Cordeiro and Lígia O. Martins
ACS Catalysis 2020, https://doi.org/10.1021/acscatal.0c01623
June 2020
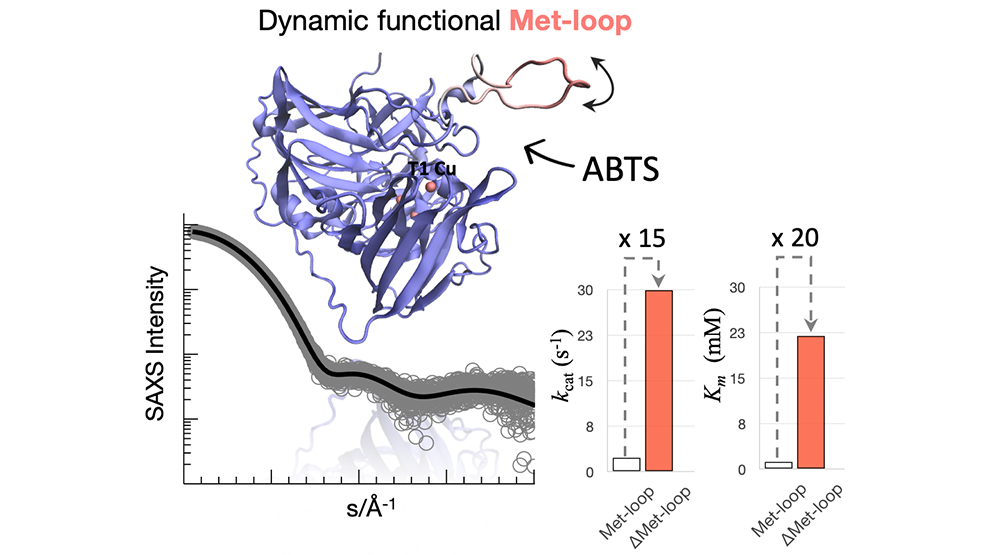
In this work, the structural features that underlie A. aeolicus McoA thermostability and catalytic promiscuity towards a wide range of substrates were characterized. A combination of mutagenesis studies performed by Vânia Brissos in LO Martins lab (Microbial and Enzyme Technology), X-ray diffraction performed by Patrícia Borges and Carlos Frazão (Structural Biology Lab), Small-angle X-ray scattering (SAXS)/ Rosetta simulations with Guillem Hernandez and Tiago Cordeiro (Dynamic Structural Biology) and molecular dynamics simulations by Zymvol Biomodeling were used. The X-ray structure of the enzyme was elucidated and a thorough analysis of the structural landscape of a 29-residue loop rich in methionines (Met-loop) invisible in the X-ray structure is presented using Rosetta and molecular dynamics simulations with ensemble-based SAXS analysis. Accurate prediction of loop structures remains challenging, especially for long segments with sizable conformational space and to the best of our knowledge, this is the first study on the conformational landscape of a low complexity Met-rich region. Loop opening and closure are known to be essential in setting up the enzyme’s active site for efficient catalysis. The performed mutagenesis results suggest that the Met-loop transient dynamic equilibrium can exert an important switch-like regulatory function, capable of controlling the access of substrates and determining the chemical environment of the enzyme’s active, defining a role for Met-rich motifs as dynamic gate-gappers.
This papers’ findings have fundamental importance to understand both MCOs functions and the role of Met-rich motifs in general. From a more technological perspective, the intrinsic plasticity of the McoA Met-loop close to the substrate-binding site makes it an attractive target for loop engineering to fine-tune the enzyme selectivity and design new hyper-thermostable enzymes for suitable biotechnological applications.
RELATED HIGHLIGHTS:
itqb.unl.pt/news/biorefinery-vision-of-the-xxi-century
itqb.unl.pt/news/new-enzyme-by-directed-evolution
Paper #3
Collaboration between Lígia O. Martins (Microbial & Enzyme Technology Lab) Smilja Todorovic (Raman BioSpectroscopy Lab) and Elin Moe (Macromolecular Crystallography Unit) @ ITQB-UNL and Marco Fraaije from @Univ of Groningen
Resonance Raman view of the active site architecture in bacterial DyP-type peroxidases
Celia M. Silveira, Elin Moe, Marco Fraaije, Lígia O. Martins and Smilja Todorovic
RSC Adv., 2020, https://doi.org/10.1039/D0RA00950D
March 2020
Dye decolorizing peroxidases (DyPs) are novel haem-containing peroxidases, which are structurally unrelated to classical peroxidases. They lack the highly conserved distal histidine that acts as an acid-base catalyst in the catalytic reaction of classical peroxidases, which implies distinct mechanistic properties. Despite the remarkable catalytic properties and recognized potential for biotechnology applications, the knowledge of DyP's structural features in solution, which govern the reactivity and catalysis, is lagging behind. Resonance Raman (RR) spectroscopy can reveal fine details of the active site structure in hemoproteins, reporting on the oxidation and spin state and coordination of the haem cofactor. We provide an overview of the haem binding pocket architecture of the enzymes from A, Band C DyP subfamilies, in the light of those established for classical peroxidases, and search for subfamily-specific features among DyPs. RR demonstrates that multiple spin populations typically co-exist in DyPs, like in the case of classical peroxidases. The haem spin/coordination state is strongly pH-dependent and correlates well with the respective catalytic properties of DyPs. Unlike in the case of classical peroxidases, a surprisingly high abundance of the catalytically incompetent low spin population is observed in several DyPs, and tentatively related to the alternative physiological function of these enzymes. The molecular details of active sites of DyPs, elucidated by RR spectroscopy, can furthermore guide approaches for biotechnological exploitation of these promising biocatalysts.
Patrícia Borges, PostDoc Researcher from ITQB-UNL attended the "1st Hands-on Course on Computational Enzyme Design" in Brno, Czech Republic
2th-5th February 2020

It was four days with a fantastic group of participants, who were eager to learn rational protein design. This interactive course provided the essential theory and hands-on experience on a number of web-based tools suitable for enzyme mining and engineering activity, specificity, enantioselectivity, stability, and solubility.
Also had the chance to discuss with the developers of these tools the optimal way to set up calculations and adequately interpret the results. Special time was devoted to the proteins of interest of the individual participants to be able to independently design proteins for academic research or commercial application.
Paper #2
Collaboration between the group of Prof. Lígia O. Martins and Smilja Todorovic lab @ ITQB-UNL
Immobilized dye-decolorizing peroxidase and directed evolution variants for hydrogen peroxide biosensing
Catarina Barbosa, Celia M. Silveira, Diogo Silva, Vânia Brissos, Peter Hildebrandt, Lígia O. Martins, Smilja Todorovic
Biosensors and Bioelectronics 2020 https://doi.org/10.1016/j.bios.2020.112055
January 2020
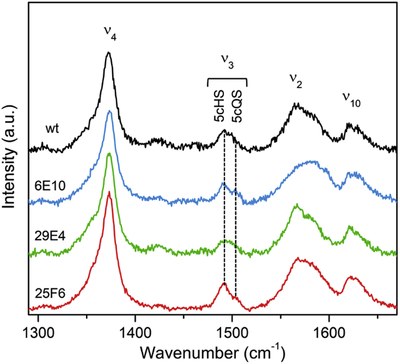
Immobilized dye-decolorizing peroxidase from Pseudomonas putida MET94 (PpDyP) and three variants generated by directed evolution (DE) are studied aiming at the design of a biosensor for H2O2 detection. Structural properties of the enzymes in solution and immobilized state are addressed by resonance Raman (RR) and surface-enhanced RR (SERR) spectroscopy, and the electrocatalytic properties are analyzed by electrochemistry. The wild-type (wt) and 29E4 variant (with E188K and H125Y mutations) represent excellent candidates for the development of H2O2 biosensors since they exhibit a good dynamic response range (1–200 μM H2O2), short response times (2 s) and a superior sensitivity (1.3–1.4 A⋅M−1⋅cm−2) for H2O2, as well as selectivity and long term stability. In contrast to the solution state, 6E10 (with E188K, A142V, and H125Y mutations) and 25F6 (with E188K, A142V, H125Y, and G129D mutations) variants display much lower activity and are inhibited by high concentrations of H2O2 upon adsorption on an electrode. In terms of sensitivity, the bioelectrodes employing wt PpDyP and 29E4 variant outperform HRP-based counterparts reported in the literature by 1–4 orders of magnitude. We propose the development of wt or 29E4 PpDyP-based biosensor as a valuable alternative to devices that rely on peroxidases.
Dissemination of the project in the Conference Microbiotec’19 at University of Coimbra, Portugal
5th-7th December 2019

The Microbiotec’19 aims to provide an international scientific forum by which researchers from all over the world share their knowledge, exchange ideas, and discuss scientific policy and regulatory issues associated with microbiology and biotechnology in the future.
The Coordinator of B-LigZymes project Professor Lígia O. Martins gave a lecture on "New Ligninolytic enzymes by directed evolutions".
The secondees of the B-LigZymes project explained the posters with the most recent achievements. The Team was composed of Professor Lígia O. Martins, Vânia Brissos, Magdalena Anna Lejmel, Carolina Rodrigues, André Taborda, Mario De Simone.
Paper #1
From the group of Professor Dimitris Argyropoulos - Departments of Chemistry & Forest Biomaterials, North Carolina State University
Are lignin-derived carbon fibers graphitic enough?
William J. Sagues, Ankush Jain, Dylan Brown, Salonika Aggarwal, Antonio Suarez, Matthew Kollman, Seonghyun Parka, and Dimitris S. Argyropoulos
Green Chem. 2019, https://doi.org/10.1039/C9GC01806A
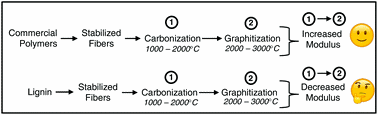
July 2019
Single component lignin-derived carbon fibers have been under development for many years, but their strength properties are still inferior to those of commercial carbon fibers. The extent of graphitization is an overlooked limitation to lignin-derived carbon fiber development, particularly for high-modulus fibers treated at high temperatures. The tensile moduli of commercial carbon fibers increase with temperature during graphitization, however, lignin-derived carbon fiber moduli stay the same or decrease. This review exposes the inability of lignin-derived carbon fibers to graphitize in a manner similar to commercial carbon fibers, thereby providing the rationale for the aforementioned discrepancy in tensile moduli-temperature trends and offering possible tangible future areas of research and development.
Dissemination of the project in the Conference BIOTRANS2019 in Groningen, NL
7th-9th July 2019
The secondees of the B-LigZymes project explained the posters with the most recent achievements. The Team was composed of Professor Lígia O. Martins, André Taborda, Magali Scocozza, Margarita Stirz, Joana Pedroso, Maria Ana Castro, Emanuele Monza, Mohamed Habib, Klara Birikh, Ulises Zitare.