Azoreductases
Annually around 800,000 tons of synthetic dyes are produced worldwide and around 10-15% of these are released into effluents. Dyes are hardly removed by conventional wastewater treatments, causing deterioration of water quality and becoming a health threat, due to their mutagenic properties. Biodegradation methods are attractive a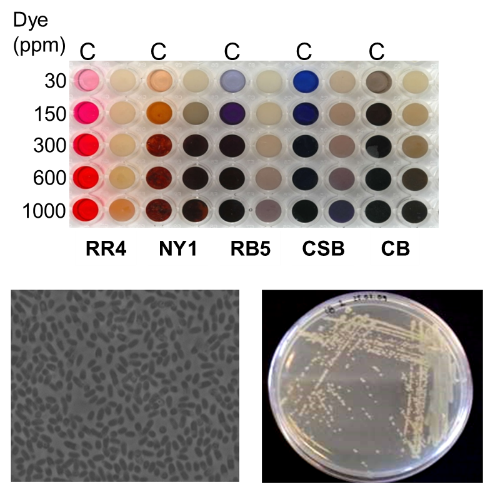 lternatives to traditional physicochemical processes, as can be less expensive, selectively achieve complete mineralization of organic pollutants without collateral destruction of natural flora and fauna. We have screened a small collection of soil microorganisms (around 150) harvested from a variety of sources for dye decolourisation. A new bacterial strain Pseudomonas putida MET94 was selected based on its high efficiency for decolourising a wide range of structurally different azo dyes (Mendes et al. 2011a). Afterwards, an azoreductase (PpAzoR) from this bacteria was cloned and expressed in E. coli and the recombinant enzyme was purified and characterized. The enzyme is a FMN-dependent NAD(P)H oxidoreductase with a ping-pong bi-bi reaction scheme showing a broad substrate specificity for azo dye reduction. The crystal structure of the PpAzoR azoredutase was determined at 1.6 Å of resolution (Gonçalves et al. 2013).
lternatives to traditional physicochemical processes, as can be less expensive, selectively achieve complete mineralization of organic pollutants without collateral destruction of natural flora and fauna. We have screened a small collection of soil microorganisms (around 150) harvested from a variety of sources for dye decolourisation. A new bacterial strain Pseudomonas putida MET94 was selected based on its high efficiency for decolourising a wide range of structurally different azo dyes (Mendes et al. 2011a). Afterwards, an azoreductase (PpAzoR) from this bacteria was cloned and expressed in E. coli and the recombinant enzyme was purified and characterized. The enzyme is a FMN-dependent NAD(P)H oxidoreductase with a ping-pong bi-bi reaction scheme showing a broad substrate specificity for azo dye reduction. The crystal structure of the PpAzoR azoredutase was determined at 1.6 Å of resolution (Gonçalves et al. 2013).
Improving the operational and thermodynamic stability of a versatile azoreductase
Enzymatic processes are environmental friendly strategies for bioremediation of effluents containing synthetic azo dyes or nitroaromatic compounds. The azoreductase PpAzoR from P. putida MET 94 is attractive for bioremediation processes; however, its low thermal kinetic stability impairs its full potential for applications. In this study, five rounds of mutagenesis and recombination followed by high-throughput screening yielded the hit variant 1B6 showing a 300-fold higher half-life at 50°C comp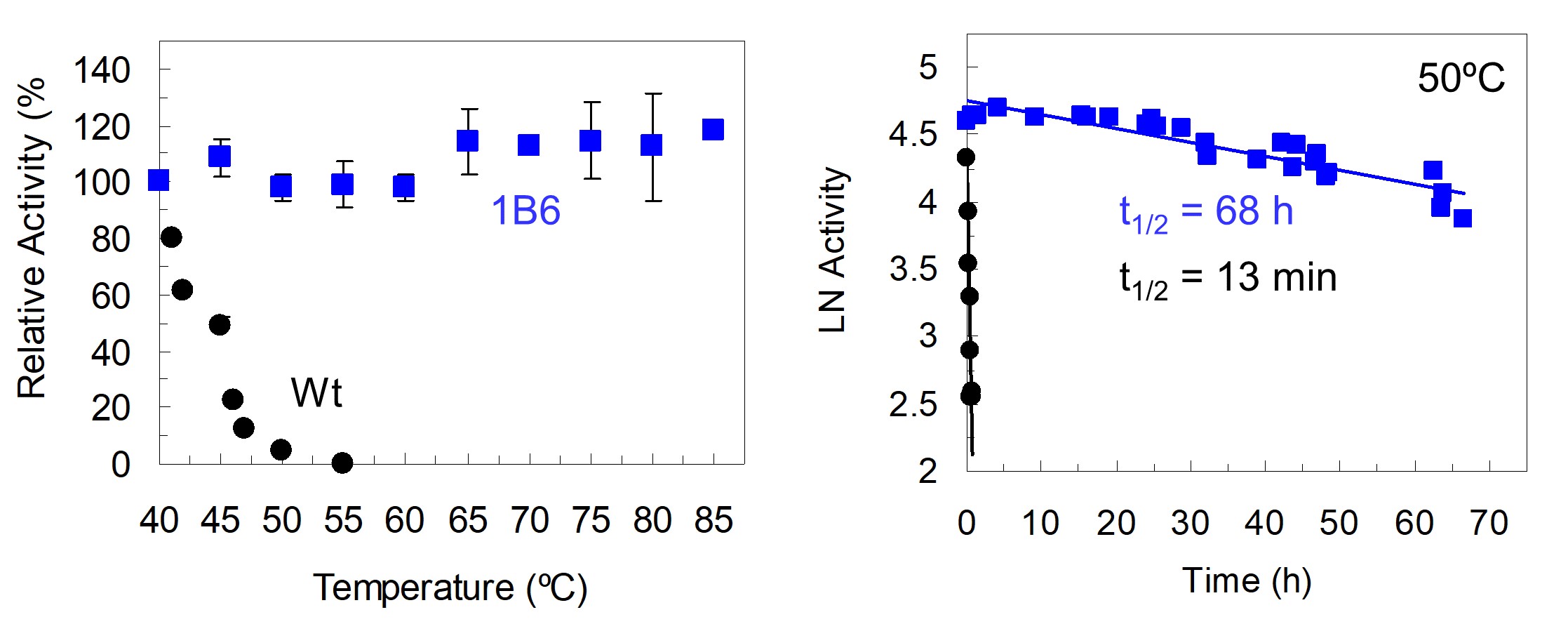 ared to the wild type enzyme (Brissos et al 2014). Two types of protein stability are of interest from a fundamental and applied perspective. Increasing the thermodynamic thermostability is the main objective when an enzyme is used in industrial applications under denaturing conditions (i.e. high temperatures or in the presence of organic solvents). For other enzymes, for example those that can be utilized for diagnostic purposes, to ensure a suitable shelf-life, it is often kinetic (or long term) stability that needs to be improved. Most frequently both stabilities correlate since increasing the enzyme resistance to the unfolding state also increases its resistance to inactivation. Remarkably, the hit variant 1B6 showed an increased resistance to aggregation as assessed by using fluorescence, calorimetry and light scattering techniques but a less stable dimeric folded state than the wild type (slightly lower melting and optimal temperature). We showed that the resistance to aggregation is mainly due to mutations that disturb hydrophobic patches and introduce surface net charges. Furthermore, we identified a variant 2A1, with 10-20°C higher melting and optimal temperatures than wild type. The increased of robustness of dimeric folded state was suggested to result from a strengthening of solvent-exposed loops and inter-dimer interactions. Overall, this work suggests that protein charge can be exploited to impart resistance to protein aggregation with implications on de novo protein design efforts, where unpredictable protein properties, including aggregation, remain a significant challenge.
ared to the wild type enzyme (Brissos et al 2014). Two types of protein stability are of interest from a fundamental and applied perspective. Increasing the thermodynamic thermostability is the main objective when an enzyme is used in industrial applications under denaturing conditions (i.e. high temperatures or in the presence of organic solvents). For other enzymes, for example those that can be utilized for diagnostic purposes, to ensure a suitable shelf-life, it is often kinetic (or long term) stability that needs to be improved. Most frequently both stabilities correlate since increasing the enzyme resistance to the unfolding state also increases its resistance to inactivation. Remarkably, the hit variant 1B6 showed an increased resistance to aggregation as assessed by using fluorescence, calorimetry and light scattering techniques but a less stable dimeric folded state than the wild type (slightly lower melting and optimal temperature). We showed that the resistance to aggregation is mainly due to mutations that disturb hydrophobic patches and introduce surface net charges. Furthermore, we identified a variant 2A1, with 10-20°C higher melting and optimal temperatures than wild type. The increased of robustness of dimeric folded state was suggested to result from a strengthening of solvent-exposed loops and inter-dimer interactions. Overall, this work suggests that protein charge can be exploited to impart resistance to protein aggregation with implications on de novo protein design efforts, where unpredictable protein properties, including aggregation, remain a significant challenge.
Synergistic action of a laccase and an azoredutase for the degradation of dye-containing effluents
We showed that although the azoreductase PpAzoR shows a significant broad specificity for the decolourisation of azo dyes, the final products of PpAzoR activity exhibited in most cases a 2 to 3-fold higher toxicity than intact dyes themselves (Mendes et al. 2011b). The addition of Cot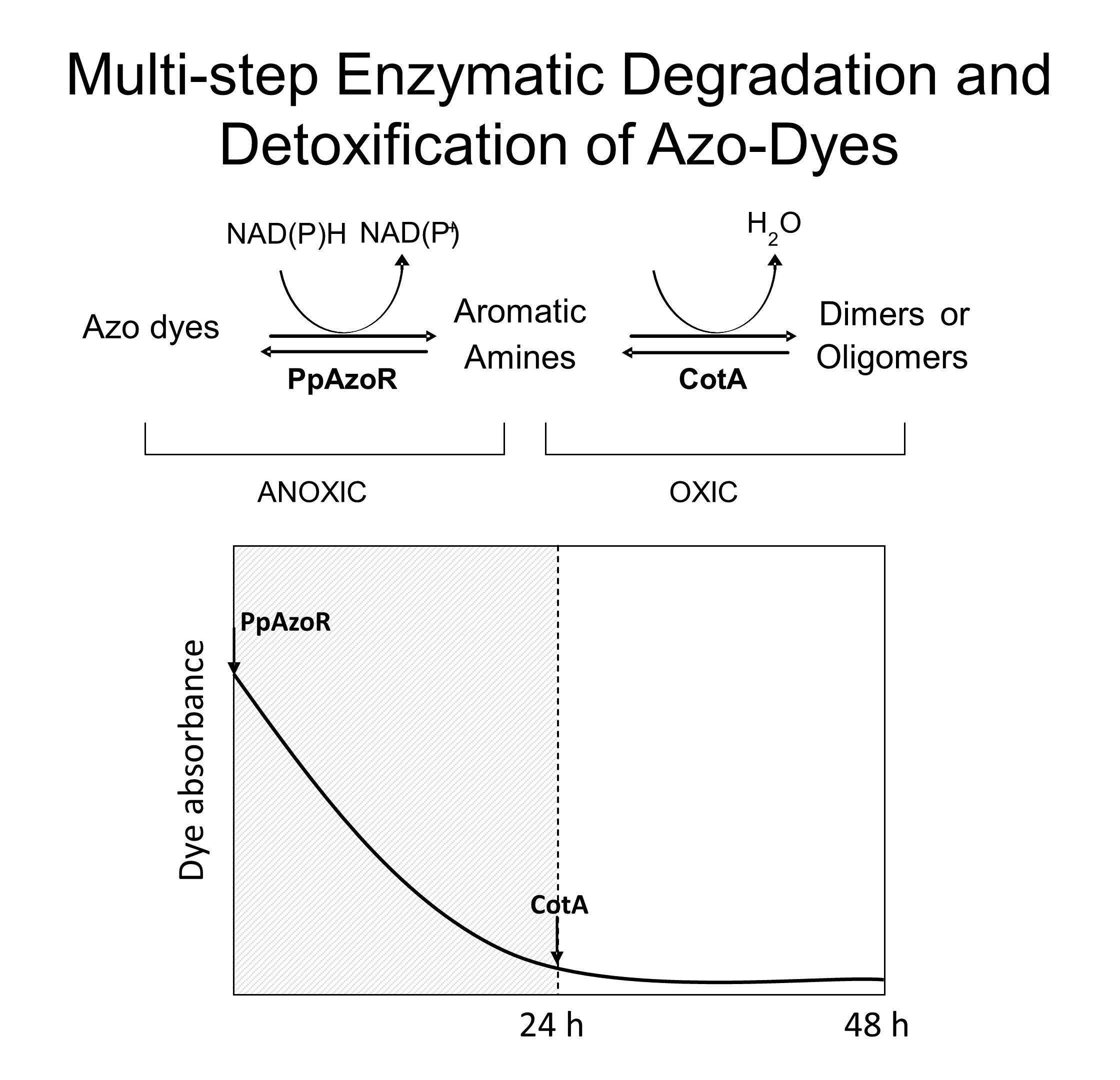 A-laccase to PpAzoR reaction mixtures lead up to 70% drop in the measured final toxicity. A sequential enzymatic process (addition of PpAzoR followed by CotA addition) was validated through the use of 18 representative azo dyes and 3 model wastewaters that mimic real dye-containing effluents. A host E. coli strain was constructed co-expressing the genes coding for both PpAzoR and CotA where the sequential action of both enzymes could be tuned by aeration conditions, since PpAzoR is active under anaerobic conditions while CotA uses molecular oxygen in the oxidation process. Whole-cell sequential enzymatic assays (static (anaerobic) followed by shaking (aerobic)) of model dye wastewaters resulted in decolourisation levels above 80% and detoxification levels up to 50%. The high attributes of this strain, make it a promising candidate for the biological degradation and detoxification treatment of industrial dye containing effluents.
A-laccase to PpAzoR reaction mixtures lead up to 70% drop in the measured final toxicity. A sequential enzymatic process (addition of PpAzoR followed by CotA addition) was validated through the use of 18 representative azo dyes and 3 model wastewaters that mimic real dye-containing effluents. A host E. coli strain was constructed co-expressing the genes coding for both PpAzoR and CotA where the sequential action of both enzymes could be tuned by aeration conditions, since PpAzoR is active under anaerobic conditions while CotA uses molecular oxygen in the oxidation process. Whole-cell sequential enzymatic assays (static (anaerobic) followed by shaking (aerobic)) of model dye wastewaters resulted in decolourisation levels above 80% and detoxification levels up to 50%. The high attributes of this strain, make it a promising candidate for the biological degradation and detoxification treatment of industrial dye containing effluents.



