DyP-type peroxidases
DyPs are a new family of heme-containing peroxidases, capable of efficiently degrading high redox aromatic compounds, including lignin-related compounds and antraquinones, and azo dyes. These enzymes are unrelated to other known peroxidases with respect to the secondary and tertiary structure and catalytic properties and are therefore very interesting from the fundamental point of view. We have cloned and characterized two new DyP type-peroxidases from Pseudomonas putida MET94 and Bacillus subtilis (Santos et al 2014).
Structural determinants of Dyps catalytic mechanism
DyPs lack the typical distal histidine of haem peroxidases and instead house an aspartate residue proposed to take the role of the His in the catalytic mechanism. We have employed site-directed mutagenesis to investigate the role of the distal pocket residues in P. putida MET94 PpDyP and B. subtilis BsDyP. We showed that the substitution of the conserved Arg catalytic residue, and not of the Asp, in the PpDyP enzyme resulted in a significant drop in reactivity (Mendes et al 2015a). This finding led to the proposal that the distal Arg acts as the acid-base catalyst in the Cpd I formation. In contrast, both Asp and Arg are important residues for the proper binding of H2O2 to the haem of BsDyP as assessed by transient and steady-state kinetics (Mendes et al 2015b). It is still not clear at this point whether the contradictory results regarding the role of the distal residues in the catalysis reflect differences in the mechanistic features of DyPs from different sources and how these differences affect their biotechnological potential; clearly, more systematic data is needed to establish the structural determinants of DyPs catalytic mechanism.
Elucidation of the catalytic mechanism of DyPs
We have elucidated for the first time the catalytic mechanism of a DyP showing that the reaction of BsDyP with hydrogen peroxide exhibits saturation behavior consistent with a two-step mecha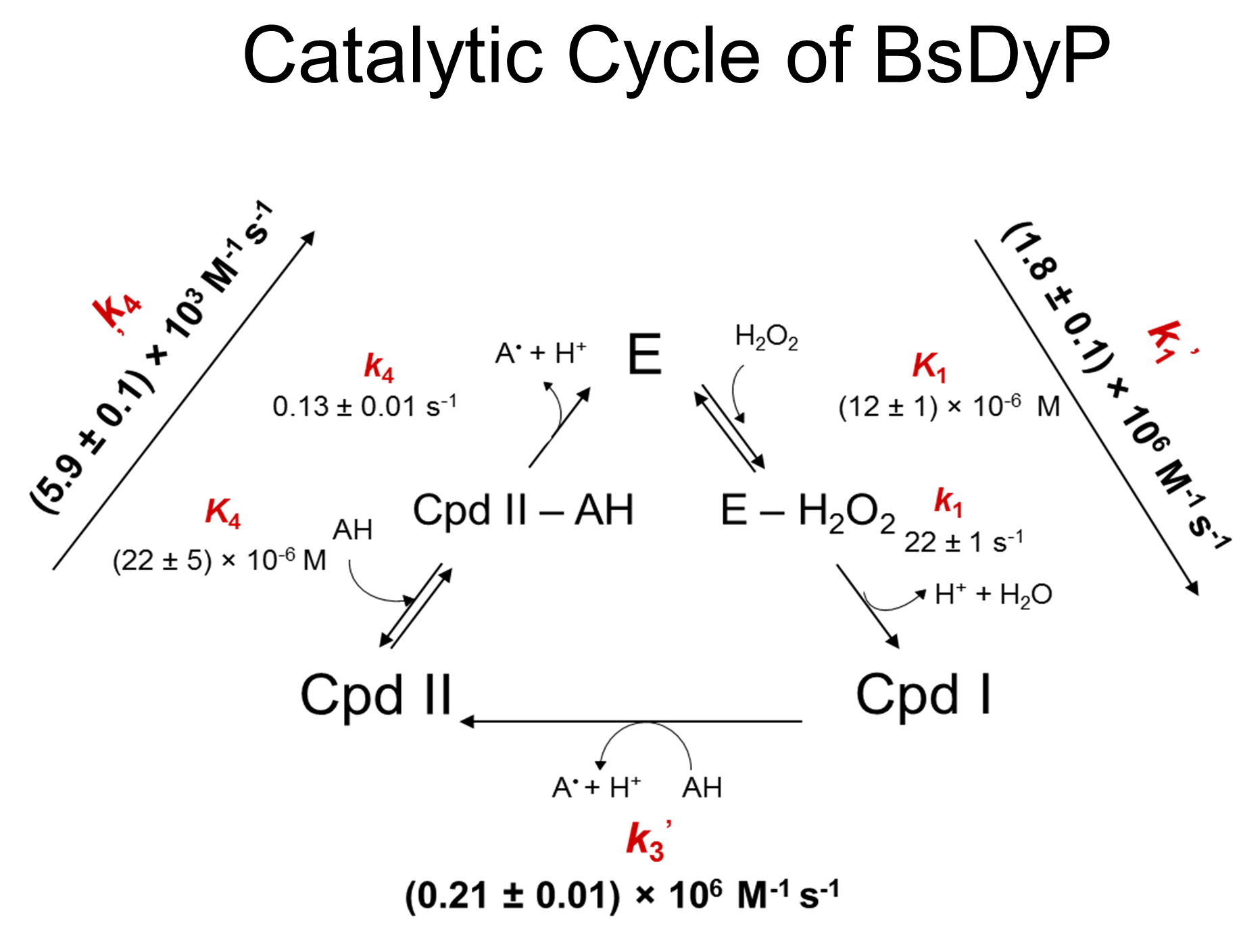 nism involving the formation of an E-H2O2 intermediate, the so-called Compound 0, followed by the formation of Compound I (Mendes et al 2015b). Interestingly, even if this result was predicted in the Poulos-Kraut model, it was previously observed in very few kinetic studies performed at low temperatures. We demonstrated that the k1obs is pH-dependent and controlled by an ionizable group with a pKa of 4.3 supporting the involvement of distal Asp. The reaction of Compound I with guaiacol obey second-order kinetics while the reaction of Compound II with guaiacol showed saturation kinetics and is the rate-limiting step in the BsDyP catalytic cycle.
nism involving the formation of an E-H2O2 intermediate, the so-called Compound 0, followed by the formation of Compound I (Mendes et al 2015b). Interestingly, even if this result was predicted in the Poulos-Kraut model, it was previously observed in very few kinetic studies performed at low temperatures. We demonstrated that the k1obs is pH-dependent and controlled by an ionizable group with a pKa of 4.3 supporting the involvement of distal Asp. The reaction of Compound I with guaiacol obey second-order kinetics while the reaction of Compound II with guaiacol showed saturation kinetics and is the rate-limiting step in the BsDyP catalytic cycle.
Improving the kinetic efficiency of a DyP peroxidase
Directed evolution was used to improve the efficiency of the bacterial PpDyP from P. putida MET94 for phenolic compounds (Brissos et al 2017). Three rounds of random mutagenesis by error-prone PCR of the ppDyP-gene followed by high-throughput screening allow identifying 6E10 variant showing 100-fold enhanced catalytic efficiency for phenolic lignin-related substrate, 2,6-dimethoxyphenol (DMP). The evolved variant showed additional improved efficiency for a number of syringyl-type phenolics, guaiacol, aromatic amines, Kraft lignin, and the lignin phenolic model dimer, guaiacylglycerol-β-guaiacyl-ether. Importantly, varia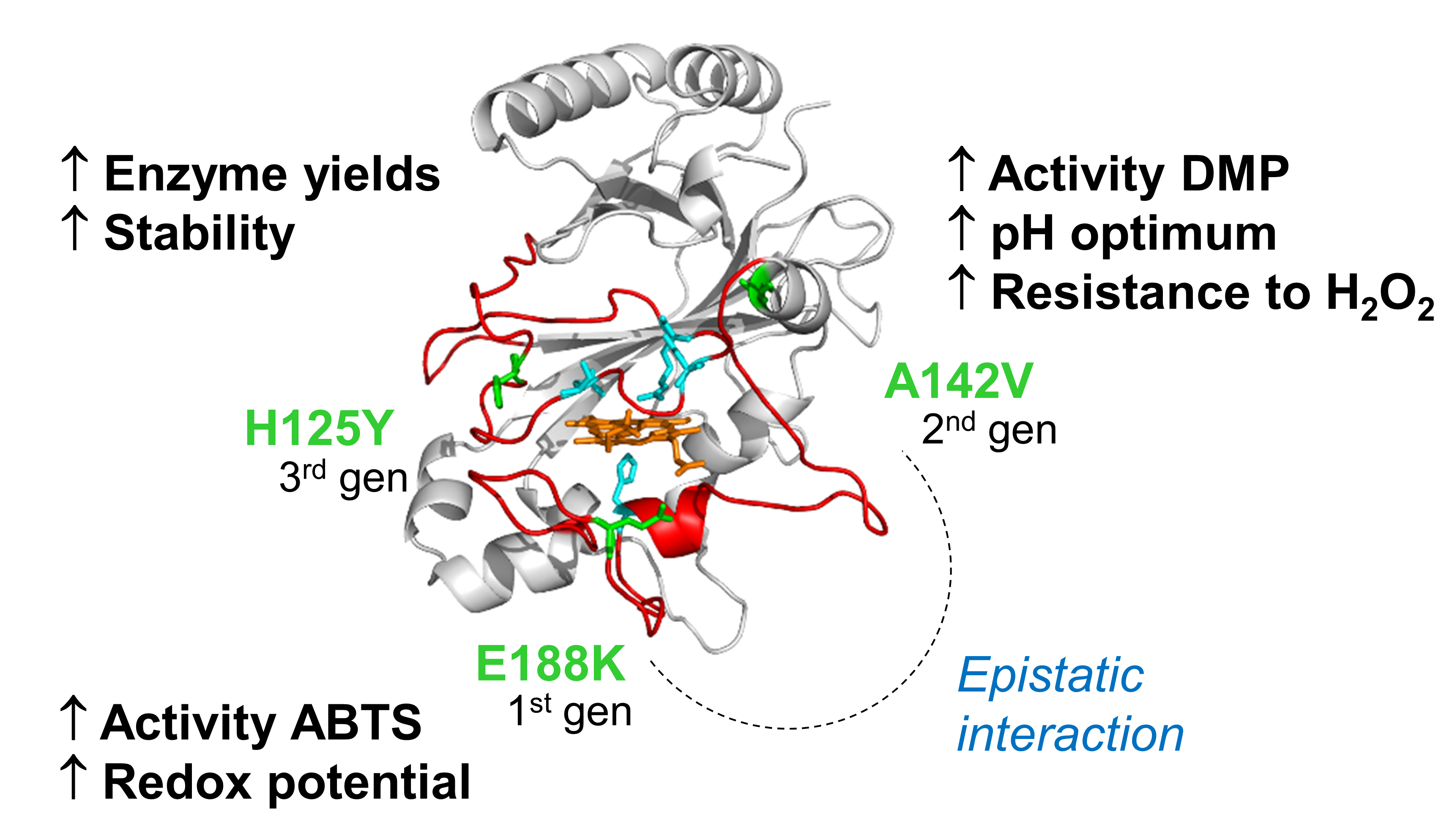 nt 6E10 displayed optimal pH at 8.5, an upshift of 4 units as compared to the wild-type, showed resistance to hydrogen peroxide inactivation, and was produced at 2-fold higher yields. The acquired mutations in the course of the evolution affected three amino acid residues (E188K, A142V, and H125Y) situated at the surface of the enzyme, in the second shell of the heme cavity. Biochemical analysis of hit variants from the laboratory evolution, and single variants constructed using site-directed mutagenesis, unveiled the critical role of acquired mutations from the catalytic, stability, and structural viewpoints (please see figure below). We show that epistasis between A142V and E188K mutations is crucial to determine the substrate specificity of 6E10. Evidence suggests that ABTS and DMP oxidation occurs at the heme access channel.
nt 6E10 displayed optimal pH at 8.5, an upshift of 4 units as compared to the wild-type, showed resistance to hydrogen peroxide inactivation, and was produced at 2-fold higher yields. The acquired mutations in the course of the evolution affected three amino acid residues (E188K, A142V, and H125Y) situated at the surface of the enzyme, in the second shell of the heme cavity. Biochemical analysis of hit variants from the laboratory evolution, and single variants constructed using site-directed mutagenesis, unveiled the critical role of acquired mutations from the catalytic, stability, and structural viewpoints (please see figure below). We show that epistasis between A142V and E188K mutations is crucial to determine the substrate specificity of 6E10. Evidence suggests that ABTS and DMP oxidation occurs at the heme access channel.



