Laccases & metalloxidases
We were pioneers in the structural and functional characterization of bacterial laccases. Laccases are green catalysts with an outstanding redox capability over a wide range of aromatic substrates using O2 as an electron acceptor and releasing water as the sole reduced product. 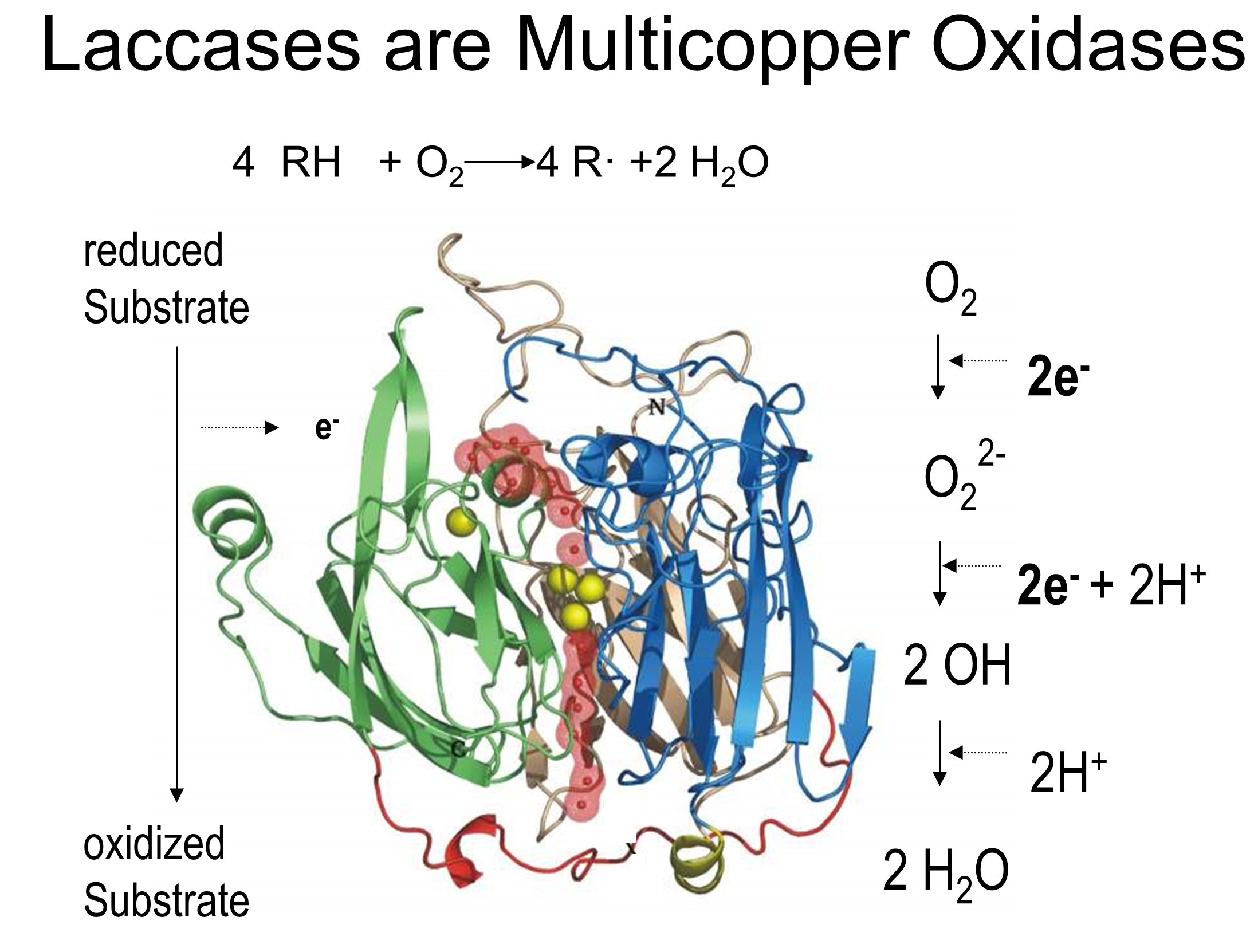 Efficient expression systems to produce large quantities of these enzymes and tools to genetically manipulate them were developed. A toolbox of experimental approaches was mounted to explore these biocatalysts, e.g. CotA-laccase was used in the degradation of synthetic dyes (Pereira et al 2009 a,b). Thorough multidisciplinary investigations of wild type and engineered variants revealed key structural and functional aspects of these enzymes; details on the substrate binding (Enguita et al 2004), solvent accessibility to catalytic centres and electrostatic interactions modulating the redox potential (Durão et al 2006), copper incorporation (Durão et al 2008, Fernandes et al 2012), and the molecular mechanism of oxygen reduction (Chen et al 2010, Brissos et al 2012).
Efficient expression systems to produce large quantities of these enzymes and tools to genetically manipulate them were developed. A toolbox of experimental approaches was mounted to explore these biocatalysts, e.g. CotA-laccase was used in the degradation of synthetic dyes (Pereira et al 2009 a,b). Thorough multidisciplinary investigations of wild type and engineered variants revealed key structural and functional aspects of these enzymes; details on the substrate binding (Enguita et al 2004), solvent accessibility to catalytic centres and electrostatic interactions modulating the redox potential (Durão et al 2006), copper incorporation (Durão et al 2008, Fernandes et al 2012), and the molecular mechanism of oxygen reduction (Chen et al 2010, Brissos et al 2012).
Copper incorporation into CotA-laccase
The standard conditions used for the overproduction of CotA-laccase in the cytoplasm of Escherichia coli result in the production of a copper depleted form of the enzyme, with negative consequences in the activity and stability of the enzyme. The identification of growth conditions that allowed for the production of fully copper loaded enzyme preparations in the cytoplasm of E. coli was therefore a hallmark in research involving bacterial laccases (Durão et al 2008). The copper content of CotA-laccase w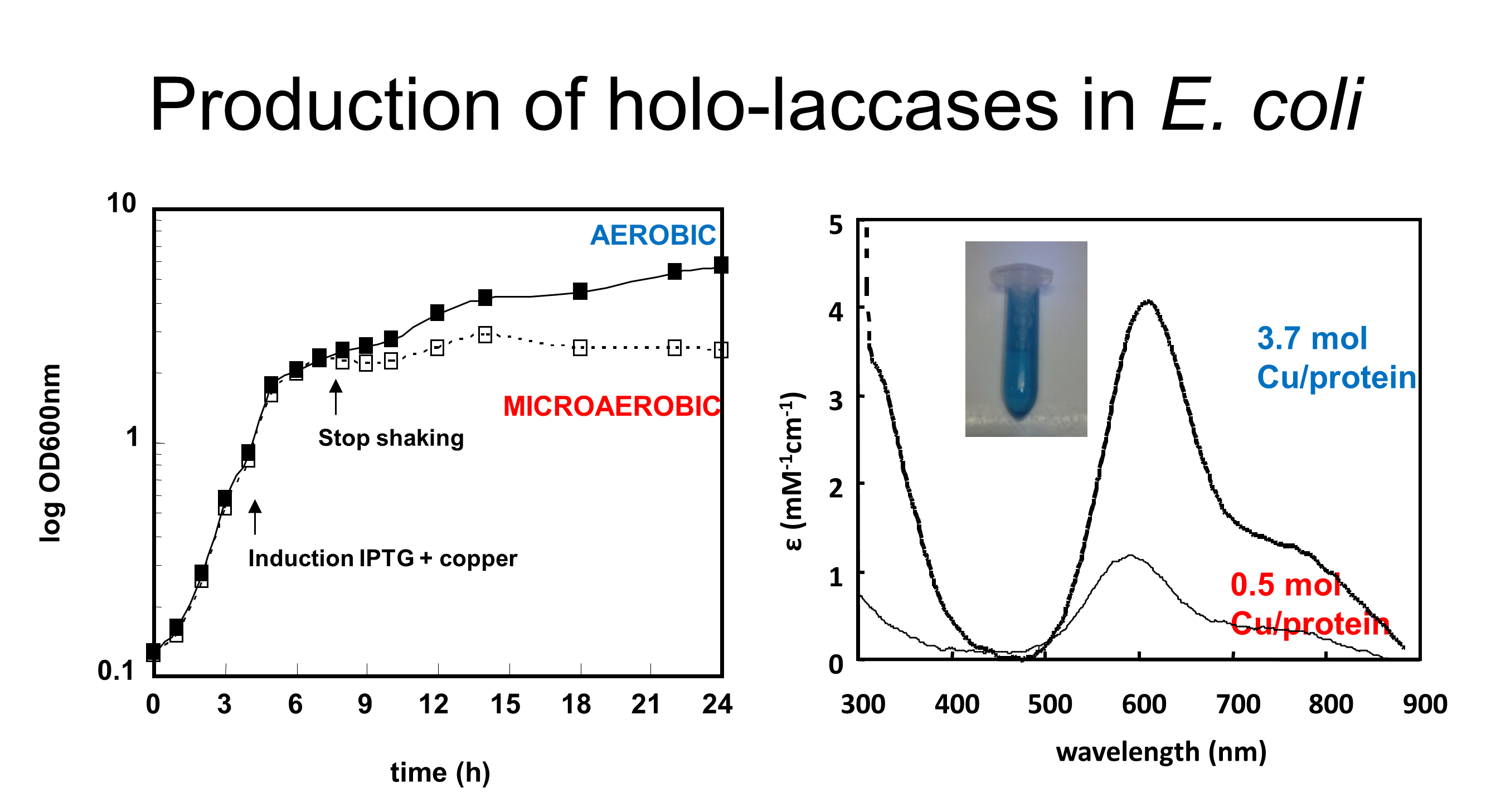 as shown to be strongly dependent on the levels of oxygen in the culture media. In copper-supplemented media, a switch from aerobic to microaerobic conditions leads to the synthesis of a recombinant holoenzyme, while the maintenance of aerobic conditions results in a copper depleted population of proteins. Strikingly, cells grown under microaerobic conditions accumulate up to 80-fold more copper than aerobic grown cells. The existence of elaborate homeostasis mechanisms in E.coli (cue and cus systems) maintain the intracellular quota for copper within a narrow range (around 10 µM), under aerobic conditions due to the well-known cytotoxicity of this transition metal, impairing the overproduction of a fully Cu-loaded enzyme. Copper incorporation was shown to be critical in the fine tuning of CotA-laccase folding in the cytoplasm of E. coli and the data obtained clearly indicated that Cu(I) is the most efficient Cu redox state to be incorporated during in vivo enzyme folding and is key to achieve a fully copper loaded and fully functional and stable enzyme (Durao et al. 2008). The incorporation of copper in CotA occurs sequentially, with the T1 site being the first to be reconstituted, followed by the T2 and T3 centers. Deeper insight into the unfolding pathway and copper incorporation in CotA was gathered through acid-induced unfolding and double-jump stopped-flow experiments (Fernandes et al. 2012). Overall the data indicates that copper has to be incorporated at later stages of in vitro folding to avoid protein aggregation (Fernandes et al. 2012) but this incorporation is critical to adjust the structure to obtain a fully functional and stable enzyme (Durao et al. 2008a).
as shown to be strongly dependent on the levels of oxygen in the culture media. In copper-supplemented media, a switch from aerobic to microaerobic conditions leads to the synthesis of a recombinant holoenzyme, while the maintenance of aerobic conditions results in a copper depleted population of proteins. Strikingly, cells grown under microaerobic conditions accumulate up to 80-fold more copper than aerobic grown cells. The existence of elaborate homeostasis mechanisms in E.coli (cue and cus systems) maintain the intracellular quota for copper within a narrow range (around 10 µM), under aerobic conditions due to the well-known cytotoxicity of this transition metal, impairing the overproduction of a fully Cu-loaded enzyme. Copper incorporation was shown to be critical in the fine tuning of CotA-laccase folding in the cytoplasm of E. coli and the data obtained clearly indicated that Cu(I) is the most efficient Cu redox state to be incorporated during in vivo enzyme folding and is key to achieve a fully copper loaded and fully functional and stable enzyme (Durao et al. 2008). The incorporation of copper in CotA occurs sequentially, with the T1 site being the first to be reconstituted, followed by the T2 and T3 centers. Deeper insight into the unfolding pathway and copper incorporation in CotA was gathered through acid-induced unfolding and double-jump stopped-flow experiments (Fernandes et al. 2012). Overall the data indicates that copper has to be incorporated at later stages of in vitro folding to avoid protein aggregation (Fernandes et al. 2012) but this incorporation is critical to adjust the structure to obtain a fully functional and stable enzyme (Durao et al. 2008a).
Changing the enzyme specificity of a hyperthermophilic metaloxidase into a laccase
The efficiency of the metallo-oxidase McoA from the hyperthermophilic bacterium Aquifex aeolicus was improved for aromatic compounds using directed evolution (Brissos et al 2015). Directed evolution is an effective and reliable approach to engineer proteins contributing simultaneously to our understanding of mechanisms of protein function and a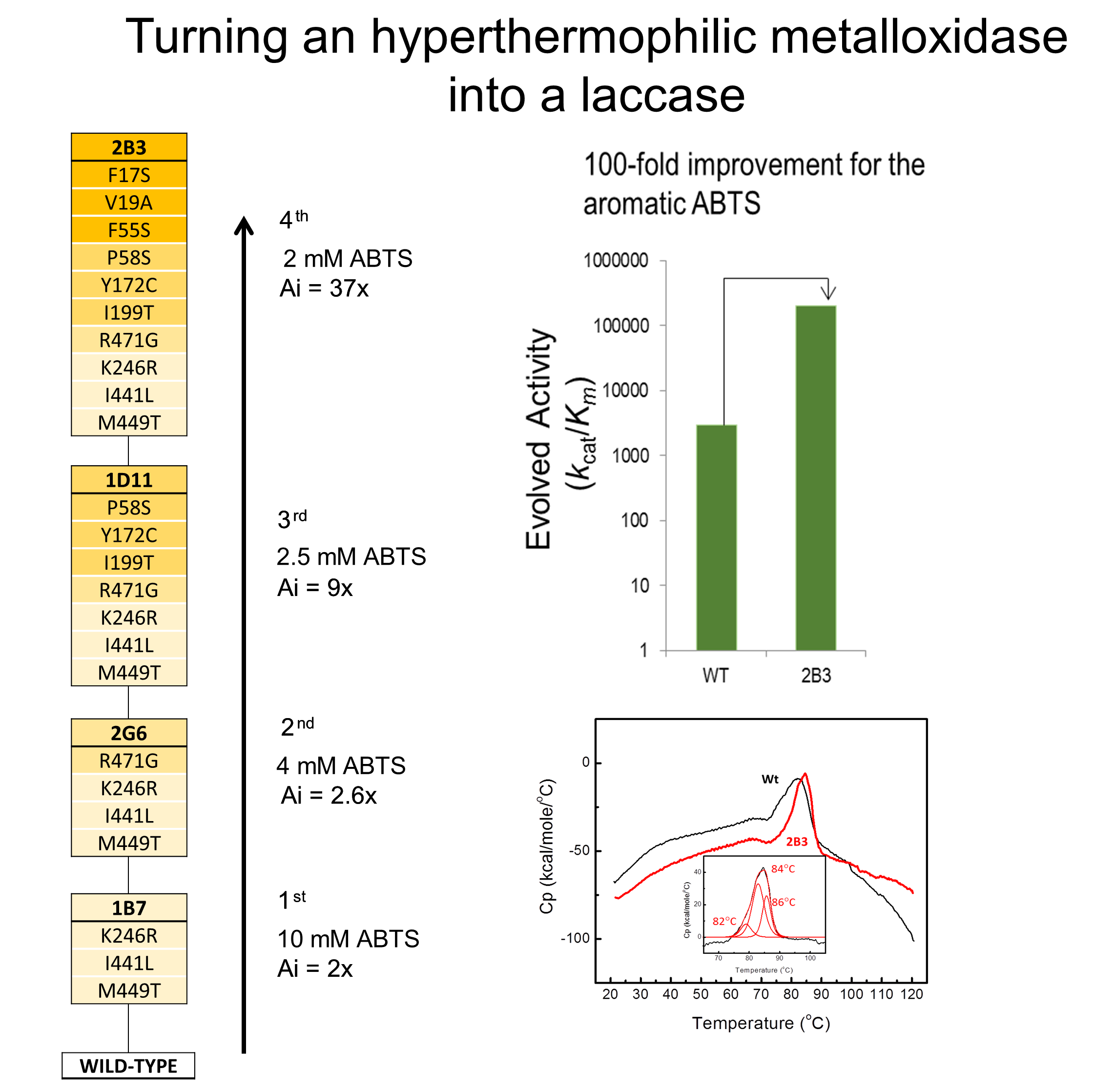 daptation. Four rounds of random mutagenesis of the mcoA-gene followed by high-throughput screening (≈ 94,000 clones) led to the identification of the 2B3 variant featuring a 2-order of magnitude higher efficiency than the wild-type enzyme for the typical laccase substrate ABTS and showing additionally a higher activity for phenolics and synthetic aromatic dyes. Notably, the recombinant 2B3 variant exhibits an enhanced solubility and thus a higher kinetic and thermodynamic thermostability as compared with the wild-type enzyme. The structural basis of the improved properties exhibited by the 2B3 variant were discussed based on studies of single and double mutations constructed using site-directed mutagenesis. The hyper-robustness of the system reported shows clear advantages for current applications and provides a powerful tool for generation of more efficient biocatalysts for specific applications.
daptation. Four rounds of random mutagenesis of the mcoA-gene followed by high-throughput screening (≈ 94,000 clones) led to the identification of the 2B3 variant featuring a 2-order of magnitude higher efficiency than the wild-type enzyme for the typical laccase substrate ABTS and showing additionally a higher activity for phenolics and synthetic aromatic dyes. Notably, the recombinant 2B3 variant exhibits an enhanced solubility and thus a higher kinetic and thermodynamic thermostability as compared with the wild-type enzyme. The structural basis of the improved properties exhibited by the 2B3 variant were discussed based on studies of single and double mutations constructed using site-directed mutagenesis. The hyper-robustness of the system reported shows clear advantages for current applications and provides a powerful tool for generation of more efficient biocatalysts for specific applications.
Mechanism of laccase reactions using phenolic compounds as redox mediators
A mechanism of lignin-related phenolics oxidation and their role in mediating non-phenolics by bacterial laccases was described (Rosado et al. 2012). The oxidation of lignin by laccases is restricted to the oxidation of phenolic lignin moiety that comprises less than 20% of the lignin polymer. This limitation has been overcome through the addition of low molecular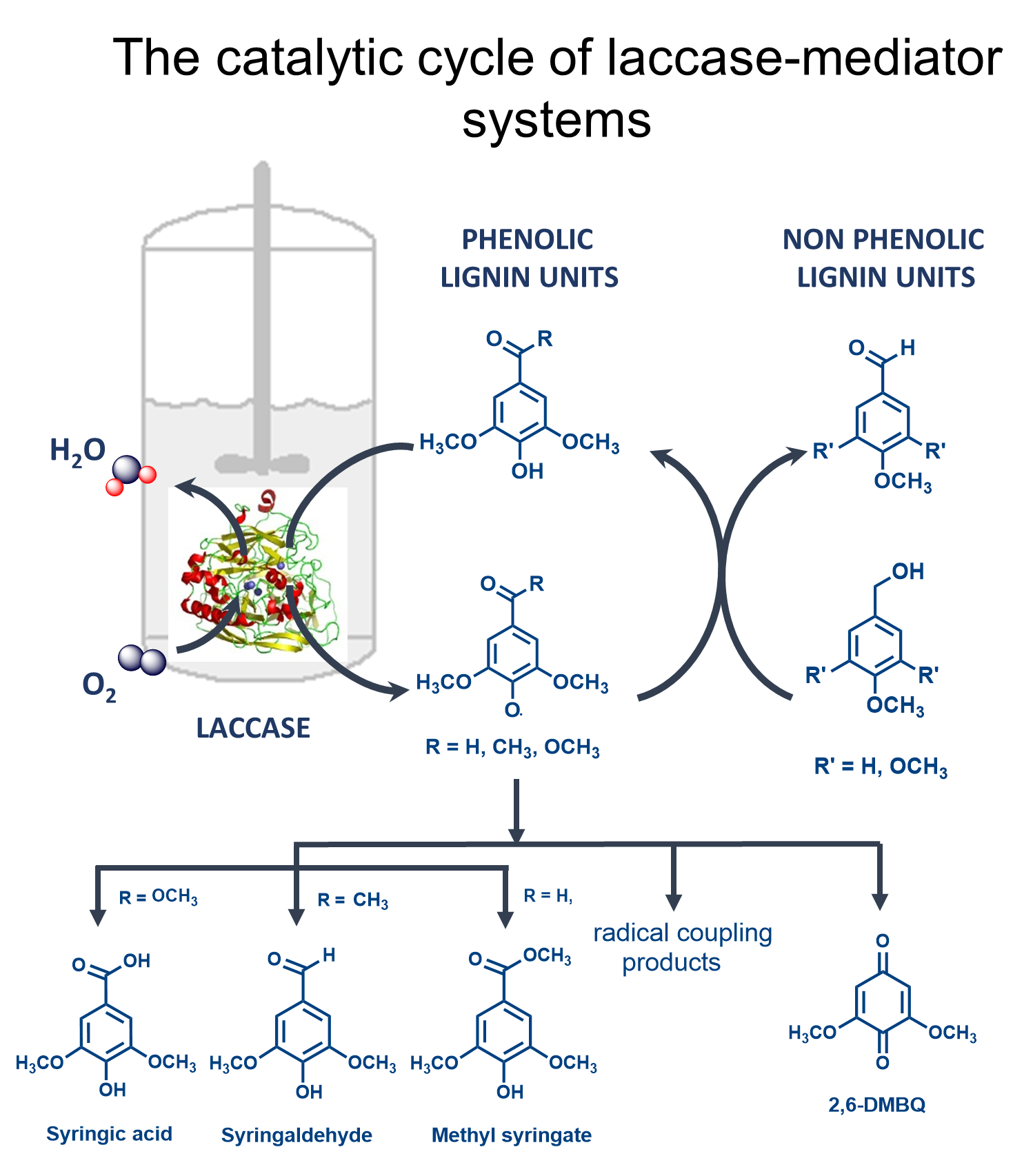 weight synthetic redox-mediators in reaction mixtures. It was reported that phenolic compounds, are capable of mediating the oxidation of non-phenolic compounds with similar or improved performances as compared to synthetic mediators (high cost and generation of toxic species). These “natural mediators” most likely represent the “true” mediators of laccase during the biodegradation of lignin in nature and can be extracted at low cost from plant materials, effluent streams of the pulp manufacture process. Firstly, we studied the pH dependence and the kinetics of oxidation of syringyl-type phenolic models using two different laccases: the low redox potential CotA-laccase and the high redox potential TvL laccase. Secondly, the efficiency of these compounds as redox mediators was tested for the oxidation of non-phenolic lignin units at different mediator:non-phenolics ratios. Finally, the products of reactions were identified using LC-MS and NMR. This strategy allow identifying the, 1) different catalytic mechanism used by bacterial and fungal laccases with implications in the optimal pH, for bacterial laccases in the neutral to alkaline, and for those of fungal origin, in the acidic range, 2) the critical importance of the chemical nature of the phenolic mediators (and not of the enzyme used) on the yields of non-phenolics conversion and, on 3) the existence of competitive routes involved in the catalytic cycle of the laccase-mediator systems.
weight synthetic redox-mediators in reaction mixtures. It was reported that phenolic compounds, are capable of mediating the oxidation of non-phenolic compounds with similar or improved performances as compared to synthetic mediators (high cost and generation of toxic species). These “natural mediators” most likely represent the “true” mediators of laccase during the biodegradation of lignin in nature and can be extracted at low cost from plant materials, effluent streams of the pulp manufacture process. Firstly, we studied the pH dependence and the kinetics of oxidation of syringyl-type phenolic models using two different laccases: the low redox potential CotA-laccase and the high redox potential TvL laccase. Secondly, the efficiency of these compounds as redox mediators was tested for the oxidation of non-phenolic lignin units at different mediator:non-phenolics ratios. Finally, the products of reactions were identified using LC-MS and NMR. This strategy allow identifying the, 1) different catalytic mechanism used by bacterial and fungal laccases with implications in the optimal pH, for bacterial laccases in the neutral to alkaline, and for those of fungal origin, in the acidic range, 2) the critical importance of the chemical nature of the phenolic mediators (and not of the enzyme used) on the yields of non-phenolics conversion and, on 3) the existence of competitive routes involved in the catalytic cycle of the laccase-mediator systems.
Green chemistry using CotA-laccase
Eco-friendly enzymatic bioprocesses represent an attractive and important alternative to the conventional chemical synthetic processes. The potential benefits of utilising biocatalysts, such as enzymes, compared to inorganic or organic catalysts, arise from their activity under mild con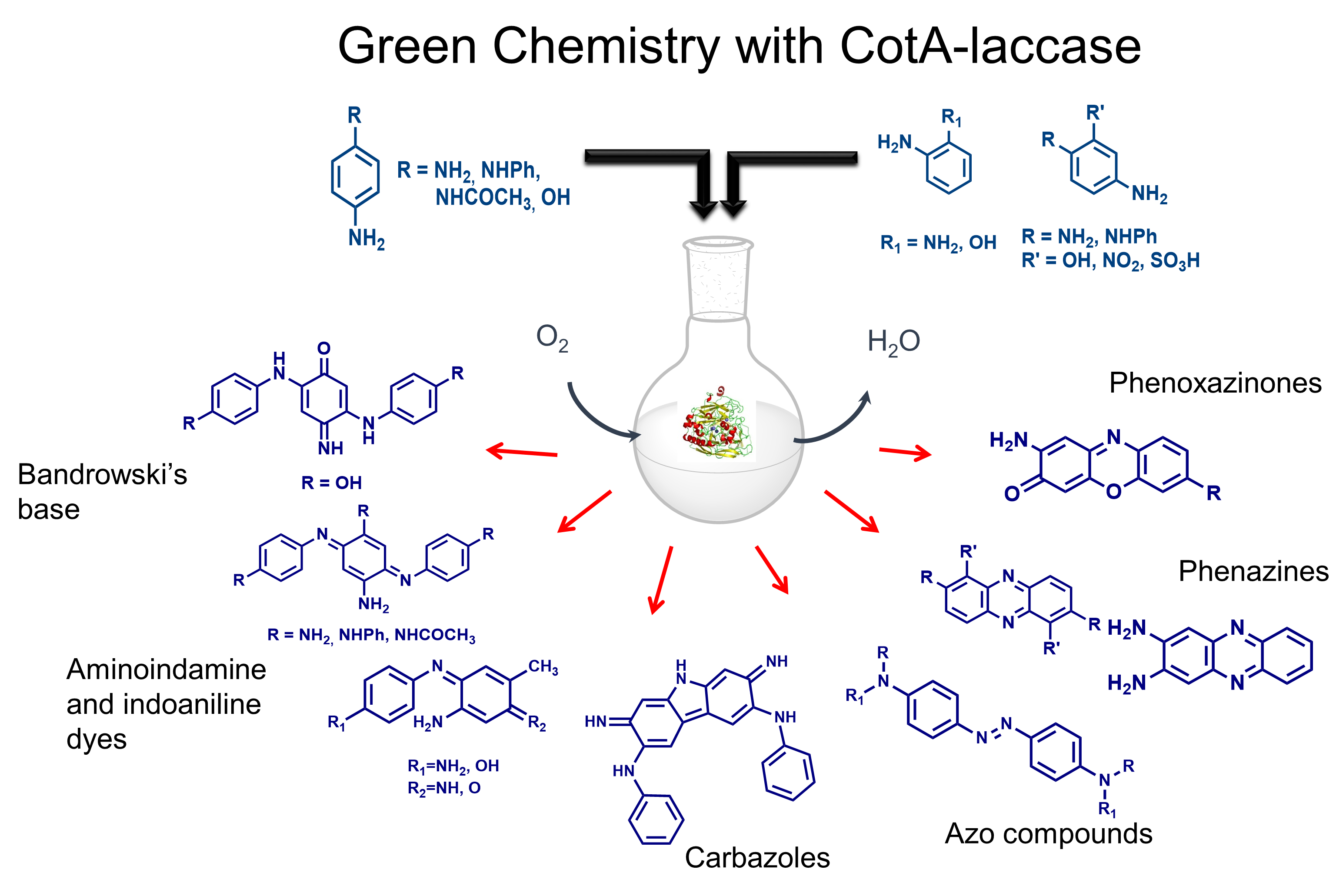 ditions of temperature, pH and pressure. Moreover, higher reaction rates and specificities are typically obtained as compared with reactions using chemical catalysts. Enzymatic conversion of aromatic amines into valuable compounds, such as phenazines and phenoxazinone derivatives, carbazoles, indo-dyes and azobenzene dyes, among others, was observed using the CotA-laccase (Sousa et al. 2014, 2016, 2018, 2019, 2020). For example, phenazines and phenoxazinones cores are multifunctional and versatile building blocks widely distributed in a vast array of biologically active compounds such as antibiotics, anti-tumor agents, pesticides, dyestuffs and biosensors. Overall, the CotA-laccase enzymatic-catalysed sequences constitute a valuable alternative to the chemical oxidative coupling of aromatic amines precursors for the production of different heterocyclic scaffolds. This work results from a close collaboration with the group of M. Paula Robalo (ISEL/IST).
ditions of temperature, pH and pressure. Moreover, higher reaction rates and specificities are typically obtained as compared with reactions using chemical catalysts. Enzymatic conversion of aromatic amines into valuable compounds, such as phenazines and phenoxazinone derivatives, carbazoles, indo-dyes and azobenzene dyes, among others, was observed using the CotA-laccase (Sousa et al. 2014, 2016, 2018, 2019, 2020). For example, phenazines and phenoxazinones cores are multifunctional and versatile building blocks widely distributed in a vast array of biologically active compounds such as antibiotics, anti-tumor agents, pesticides, dyestuffs and biosensors. Overall, the CotA-laccase enzymatic-catalysed sequences constitute a valuable alternative to the chemical oxidative coupling of aromatic amines precursors for the production of different heterocyclic scaffolds. This work results from a close collaboration with the group of M. Paula Robalo (ISEL/IST).



