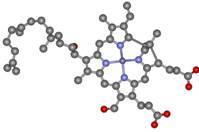
 Heme
proteins Heme
proteins
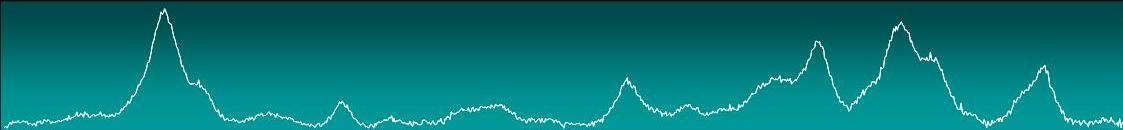
Resonance
Raman spectra of heme proteins are highly specific
and sensitive, unambiguously revealing the type,
redox, coordination and spin state of a heme group, as well as the
interactions/ processes that can modify some of these features.
Special
emphasis in our research is paid to heme
copper oxygen reductases, terminal enzymes in
the
respiratory chains of various bacteria and
archeae.
Heme-copper oxygen reductases reduce oxygen to water. The catalytic
reaction is coupled to the translocation of the protons across the
membrane, creating an electrochemical proton gradient that drives ATP
synthesis. In spite of the major role of the terminal oxidases in the
oxygen consumption by living organisms, the mechanism of their
function, the coupling between the redox processes and proton
translocation is not well understood yet. A prerequisite for
understanding the functioning of these complex multi-center redox
enzymes is determination of individual midpoint potentials and their
possible coupling.
SERR spectroelectrochemical titrations
allow
determination of the redox potentials of different heme groups for
protein molecules immobilized on nanostructured silver electrodes,
under conditions that mimic some features of the physiological
membrane, such as: hydrophobic environment, restricted motion and
defined directionality of electron transfer. Also, RR and SERR can
reveal differences in terms of catalytic cycle, regulation of electron
flow and proton translocation between the oxidases of different types.

|